Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood-Brain Barrier Permeation
- PMID: 25729344
- PMCID: PMC4340520
- DOI: 10.1002/adfm.201401199
Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood-Brain Barrier Permeation
Abstract
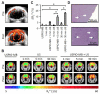
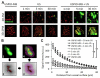
Efficient and safe drug delivery across the blood-brain barrier (BBB) remains to be one of the major challenges of biomedical and (nano-) pharmaceutical research. Here, we show that poly(butyl cyanoacrylate)-based microbubbles (MB), carrying ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles within their shell, can be used to mediate and monitor BBB permeation. Upon exposure to transcranial ultrasound pulses, USPIO-MB are destroyed, resulting in acoustic forces inducing vessel permeability. At the same time, USPIO are released from the MB shell, they extravasate across the permeabilized BBB and they accumulate in extravascular brain tissue, thereby providing non-invasive R2*-based magnetic resonance imaging information on the extent of BBB opening. Quantitative changes in R2* relaxometry were in good agreement with 2D and 3D microscopy results on the extravascular deposition of the macromolecular model drug FITC-dextran into the brain. Such theranostic materials and methods are considered to be useful for mediating and monitoring drug delivery across the BBB, and for enabling safe and efficient treatment of CNS disorders.
Keywords: Drug Delivery; Hybrid Materials; Magnetic Nanoparticles; Medical Applications; Stimuli-Responsive Materials.
Figures


References
-
- Miller G. Science. 2002;297:1116–1118. - PubMed
-
- Pardridge WM. Drug Discov. Today. 2007;12:54–61. - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E. Nat. Rev. Neurosci. 2006;7:41–53. - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Nature. 2010;468:557–561. - PubMed
-
- Brown RC, Egleton RD, Davis TP. Brain Res. 2004;1014:221–227. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
