The inner CSF-brain barrier: developmentally controlled access to the brain via intercellular junctions
- PMID: 25729345
- PMCID: PMC4325900
- DOI: 10.3389/fnins.2015.00016
The inner CSF-brain barrier: developmentally controlled access to the brain via intercellular junctions
Abstract
In the adult the interface between the cerebrospinal fluid and the brain is lined by the ependymal cells, which are joined by gap junctions. These intercellular connections do not provide a diffusional restrain between the two compartments. However, during development this interface, initially consisting of neuroepithelial cells and later radial glial cells, is characterized by "strap" junctions, which limit the exchange of different sized molecules between cerebrospinal fluid and the brain parenchyma. Here we provide a systematic study of permeability properties of this inner cerebrospinal fluid-brain barrier during mouse development from embryonic day, E17 until adult. Results show that at fetal stages exchange across this barrier is restricted to the smallest molecules (286Da) and the diffusional restraint is progressively removed as the brain develops. By postnatal day P20, molecules the size of plasma proteins (70 kDa) diffuse freely. Transcriptomic analysis of junctional proteins present in the cerebrospinal fluid-brain interface showed expression of adherens junctional proteins, actins, cadherins and catenins changing in a development manner consistent with the observed changes in the permeability studies. Gap junction proteins were only identified in the adult as was claudin-11. Immunohistochemistry was used to localize at the cellular level some of the adherens junctional proteins of genes identified from transcriptomic analysis. N-cadherin, β - and α-catenin immunoreactivity was detected outlining the inner CSF-brain interface from E16; most of these markers were not present in the adult ependyma. Claudin-5 was present in the apical-most part of radial glial cells and in endothelial cells in embryos, but only in endothelial cells including plexus endothelial cells in adults. Claudin-11 was only immunopositive in the adult, consistent with results obtained from transcriptomic analysis. These results provide information about physiological, molecular and morphological-related permeability changes occurring at the inner cerebrospinal fluid-brain barrier during brain development.
Keywords: barriers; brain development; cerebrospinal fluid; permeability; ventricular zone.
Figures
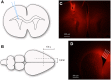



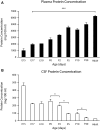

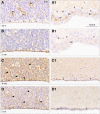
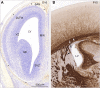
Similar articles
-
Mechanisms that determine the internal environment of the developing brain: a transcriptomic, functional and ultrastructural approach.PLoS One. 2013 Jul 2;8(7):e65629. doi: 10.1371/journal.pone.0065629. Print 2013. PLoS One. 2013. PMID: 23843944 Free PMC article.
-
The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system?F1000Res. 2016 Mar 10;5:F1000 Faculty Rev-313. doi: 10.12688/f1000research.7378.1. eCollection 2016. F1000Res. 2016. PMID: 26998242 Free PMC article. Review.
-
Phorbol ester induced changes in tight and adherens junctions in the choroid plexus epithelium and in the ependyma.Brain Res. 2000 Jan 31;854(1-2):197-206. doi: 10.1016/s0006-8993(99)02355-0. Brain Res. 2000. PMID: 10784122
-
Barrier mechanisms in the brain, II. Immature brain.Clin Exp Pharmacol Physiol. 1999 Feb;26(2):85-91. doi: 10.1046/j.1440-1681.1999.02987.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10065326 Review.
-
Blood-brain, blood-cerebrospinal fluid and cerebrospinal fluid-brain barriers in a marsupial (Macropus eugenii) during development.J Physiol. 1988 Sep;403:367-88. doi: 10.1113/jphysiol.1988.sp017254. J Physiol. 1988. PMID: 3075668 Free PMC article.
Cited by
-
The development and function of the brain barriers - an overlooked consideration for chemical toxicity.Front Toxicol. 2022 Oct 18;4:1000212. doi: 10.3389/ftox.2022.1000212. eCollection 2022. Front Toxicol. 2022. PMID: 36329715 Free PMC article. Review.
-
Facilitating drug delivery in the central nervous system by opening the blood-cerebrospinal fluid barrier with a single low energy shockwave pulse.Fluids Barriers CNS. 2022 Jan 6;19(1):3. doi: 10.1186/s12987-021-00303-x. Fluids Barriers CNS. 2022. PMID: 34991647 Free PMC article.
-
Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy.J Clin Med. 2019 Jan 24;8(2):137. doi: 10.3390/jcm8020137. J Clin Med. 2019. PMID: 30682787 Free PMC article.
-
A pilot study on the use of cerebrospinal fluid cell-free DNA in intramedullary spinal ependymoma.J Neurooncol. 2017 Oct;135(1):29-36. doi: 10.1007/s11060-017-2557-y. Epub 2017 Sep 12. J Neurooncol. 2017. PMID: 28900844 Free PMC article.
-
Thyroid hormone action controls multiple components of cell junctions at the ventricular zone in the newborn rat brain.Front Endocrinol (Lausanne). 2023 Feb 10;14:1090081. doi: 10.3389/fendo.2023.1090081. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843608 Free PMC article.
References
-
- Adinolfi M. (1985). The development of the human blood-CSF-brain barrier. Dev. Med. Child Neurol. 27, 532–537. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

