Cilia in the choroid plexus: their roles in hydrocephalus and beyond
- PMID: 25729351
- PMCID: PMC4325912
- DOI: 10.3389/fncel.2015.00039
Cilia in the choroid plexus: their roles in hydrocephalus and beyond
Abstract
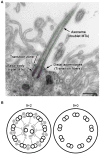
Cilia are whip-like projections that are widely conserved in eukaryotes and function as a motile propeller and/or sensory platform to detect various extracellular stimuli. In vertebrates, cilia are ubiquitously found in most cells, showing structural and functional diversities depending on the cell type. In this review, we focus on the structure and function of cilia in choroid plexus epithelial cells (CPECs). CPECs form one or two dozen non-motile 9+0 cilia, which display transient acquisition of motility during development. Genetic malfunction of cilia can lead to failure of multiple organs including the brain. Especially, several groups have demonstrated that the defects in CPEC cilia cause the communicating form of hydrocephalus. In order to elucidate the molecular mechanisms underlying the hydrocephalus, we have previously demonstrated that the cilia possess an NPFF receptor for autocrine signaling to regulate transepithelial fluid transport. In this perspective, we also discuss the potential involvement of cilia in the other aspects of choroid plexus functions, such as the regulation of brain development and neuroinflammation.
Keywords: cerebrospinal fluid; cilia; diversity; hydrocephalus; multiciliogenesis.
Figures

References
-
- Banizs B., Komlosi P., Bevensee M. O., Schwiebert E. M., Bell P. D., Yoder B. K. (2007). Altered pH(i) regulation and Na(+)/HCO3(-) transporter activity in choroid plexus of cilia-defective Tg737(orpk) mutant mouse. Am. J. Physiol. Cell Physiol. 292, C1409–C1416. 10.1152/ajpcell.00408.2006 - DOI - PubMed
-
- Benadiba C., Magnani D., Niquille M., Morle L., Valloton D., Nawabi H., et al. . (2012). The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development. PLoS Genet. 8:e1002606. 10.1371/journal.pgen.1002606 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

