Synthetic biodegradable functional polymers for tissue engineering: a brief review
- PMID: 25729390
- PMCID: PMC4341840
- DOI: 10.1007/s11426-014-5086-y
Synthetic biodegradable functional polymers for tissue engineering: a brief review
Abstract
Scaffolds play a crucial role in tissue engineering. Biodegradable polymers with great processing flexibility are the predominant scaffolding materials. Synthetic biodegradable polymers with well-defined structure and without immunological concerns associated with naturally derived polymers are widely used in tissue engineering. The synthetic biodegradable polymers that are widely used in tissue engineering, including polyesters, polyanhydrides, polyphosphazenes, polyurethane, and poly (glycerol sebacate) are summarized in this article. New developments in conducting polymers, photoresponsive polymers, amino-acid-based polymers, enzymatically degradable polymers, and peptide-activated polymers are also discussed. In addition to chemical functionalization, the scaffold designs that mimic the nano and micro features of the extracellular matrix (ECM) are presented as well, and composite and nanocomposite scaffolds are also reviewed.
Keywords: functional polymers; scaffolds; synthetic biodegradable polymers; tissue engineering.
Figures

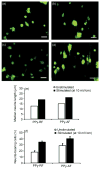
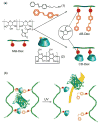

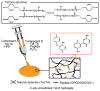


References
-
- Couch N, Wtlson R, Hager E, Murray J. Transplantatton of cadaver kidneys experience with 21 cases. Surgery. 1966;59:183–188. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Guo BL, Glavas L, Albertsson AC. Biodegradable and electrically conducting polymers for biomedical applications. Prog Polym Sci. 2013;38:1263–1286.
-
- Liu XH, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12:911–919. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
