Adjuvant chemotherapy for resected early-stage non-small cell lung cancer
- PMID: 25730344
- PMCID: PMC10542092
- DOI: 10.1002/14651858.CD011430
Adjuvant chemotherapy for resected early-stage non-small cell lung cancer
Abstract
Background: To evaluate the effects of administering chemotherapy following surgery, or following surgery plus radiotherapy (known as adjuvant chemotherapy) in patients with early stage non-small cell lung cancer (NSCLC),we performed two systematic reviews and meta-analyses of all randomised controlled trials using individual participant data. Results were first published in The Lancet in 2010.
Objectives: To compare, in terms of overall survival, time to locoregional recurrence, time to distant recurrence and recurrence-free survival:A. Surgery versus surgery plus adjuvant chemotherapyB. Surgery plus radiotherapy versus surgery plus radiotherapy plus adjuvant chemotherapyin patients with histologically diagnosed early stage NSCLC.(2)To investigate whether or not predefined patient subgroups benefit more or less from cisplatin-based chemotherapy in terms of survival.
Search methods: We supplemented MEDLINE and CANCERLIT searches (1995 to December 2013) with information from trial registers, handsearching relevant meeting proceedings and by discussion with trialists and organisations.
Selection criteria: We included trials of a) surgery versus surgery plus adjuvant chemotherapy; and b) surgery plus radiotherapy versus surgery plus radiotherapy plus adjuvant chemotherapy, provided that they randomised NSCLC patients using a method which precluded prior knowledge of treatment assignment.
Data collection and analysis: We carried out a quantitative meta-analysis using updated information from individual participants from all randomised trials. Data from all patients were sought from those responsible for the trial. We obtained updated individual participant data (IPD) on survival, and date of last follow-up, as well as details of treatment allocated, date of randomisation, age, sex, histological cell type, stage, and performance status. To avoid potential bias, we requested information for all randomised patients, including those excluded from the investigators' original analyses. We conducted all analyses on intention-to-treat on the endpoint of survival. For trials using cisplatin-based regimens, we carried out subgroup analyses by age, sex, histological cell type, tumour stage, and performance status.
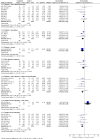
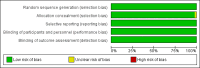
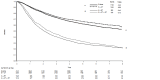
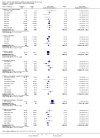
Main results: We identified 35 trials evaluating surgery plus adjuvant chemotherapy versus surgery alone. IPD were available for 26 of these trials and our analyses are based on 8447 participants (3323 deaths) in 34 trial comparisons. There was clear evidence of a benefit of adding chemotherapy after surgery (hazard ratio (HR)= 0.86, 95% confidence interval (CI)= 0.81 to 0.92, p< 0.0001), with an absolute increase in survival of 4% at five years.We identified 15 trials evaluating surgery plus radiotherapy plus chemotherapy versus surgery plus radiotherapy alone. IPD were available for 12 of these trials and our analyses are based on 2660 participants (1909 deaths) in 13 trial comparisons. There was also evidence of a benefit of adding chemotherapy to surgery plus radiotherapy (HR= 0.88, 95% CI= 0.81 to 0.97, p= 0.009). This represents an absolute improvement in survival of 4% at five years.For both meta-analyses, we found similar benefits for recurrence outcomes and there was little variation in effect according to the type of chemotherapy, other trial characteristics or patient subgroup.We did not undertake analysis of the effects of adjuvant chemotherapy on quality of life and adverse events. Quality of life information was not routinely collected during the trials, but where toxicity was assessed and mentioned in the publications, it was thought to be manageable. We considered the risk of bias in the included trials to be low.
Authors' conclusions: Results from 47 trial comparisons and 11,107 patients demonstrate the clear benefit of adjuvant chemotherapy for these patients, irrespective of whether chemotherapy was given in addition to surgery or surgery plus radiotherapy. This is the most up-to-date and complete systematic review and individual participant data (IPD) meta-analysis that has been carried out.
Conflict of interest statement
There is no known conflict of interest.
Figures







References
References to studies included in this review
A01 IPCR, Chiba {published and unpublished data}
-
- Kimura H, Yamaguchi Y, Fujisawa T, Baba M, Shiba M. A randomized controlled study of postoperative adjuvant chemoimmunotherapy of resected non‐small cell lung cancer with IL2 and LAK cells. Lung Cancer 1991;7(Suppl):113.
A02 JLCSSG {published and unpublished data}
-
- Ohta M, Tsuchiya R, Shimoyama M, Sawamura K, Mori T, Miyazawa N, et al. Adjuvant chemotherapy for completely resected stage III non‐small cell lung cancer. Journal of Thoracic and Cardiovascular Surgery 1993;106:703‐8. - PubMed
A03 Mineo {published and unpublished data}
-
- Mineo TC, Ambrogi V, Corsaro V, Roselli M. Postoperative adjuvant therapy for stage IB non‐small cell lung cancer. European Journal of Cardio‐Thoracic Surgery 2001;20(2):378‐84. - PubMed
A04 Park1 {published and unpublished data}
-
- Park JH, Lee C‐T, Lee HW, Baek HJ, Zo JI, Shim YM. Postoperative adjuvant chemotherapy for stage I non‐small cell lung cancer. European Journal of Cardio‐Thoracic Surgery 2005;27:1086‐91. - PubMed
A05 Park2 {published and unpublished data}
-
- Park JH. Postoperative adjuvant therapy for stage IIIA non‐small cell lung cancer. Journal of Thoracic Oncology 2007;2(8 Suppl 4):S651.
A06 ALPI1 {published and unpublished data}
-
- Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II or IIIa non‐small cell lung cancer. Journal of the National Cancer Institute 2003;95(19):1453‐61. - PubMed
A07 IALT1 {published and unpublished data}
-
- Arriagada R, Dunant A, Pignon J‐P, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. Journal of Clinical Oncology 2010;28:35‐42. - PubMed
-
- The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small cell lung cancer. New England Journal of Medicine 2004;350:351‐60. - PubMed
A08 BLT1 {published and unpublished data}
-
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MKB, et al. Chemotherapy for patients with non‐small cell lung cancer: the surgical setting of the Big Lung Trial. European Journal of Cardio‐Thoracic Surgery 2004;26:173‐82. - PubMed
A09 JCOG 9304 {published and unpublished data}
-
- Tada H, Tsuchiya R, Ichinose Y, Koike T, Nishizawa N, Nagai K, et al. A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non‐small cell lung cancer (JCOG 9304). Lung Cancer 2004;43:167‐73. - PubMed
A10 ANITA1 {published and unpublished data}
-
- Douillard JY, Rosell R, Lena M, Carpagnano F, Ramlau R, Gonzales‐Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB‐IIIA non‐small‐cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncology 2006;7:719‐27. - PubMed
A11 JBR10 {published and unpublished data}
-
- Butts CA, Ding K, Seymour L, Twumasi‐Ankrah P, Graham B, Gandara D, et al. Randomised phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage Ib and II non‐small cell lung cancer: Updated survival analysis of JBR‐10. Journal of Clinical Oncology 2010;28:29‐34. - PMC - PubMed
-
- Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs observation in resected non‐small cell lung cancer. New England Journal of Medicine 2005;352:2589‐97. - PubMed
A12 IALT2 {published and unpublished data}
-
- Arriagada R, Dunant A, Pignon J‐P, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. Journal of Clinical Oncology 2010;28:35‐42. - PubMed
-
- The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small cell lung cancer. New England Journal of Medicine 2004;350:351‐60. - PubMed
A13 BLT2 {published and unpublished data}
-
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MKB, et al. Chemotherapy for patients with non‐small cell lung cancer: the surgical setting of the Big Lung Trial. European Journal of Cardio‐Thoracic Surgery 2004;26:173‐82. - PubMed
A14 CALGB 9633 {published and unpublished data}
-
- Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non‐small‐cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. Journal of Clinical Oncology 2008;26(31):5043‐51. - PMC - PubMed
A15 LCSG 801 {published and unpublished data}
-
- Feld R, Rubinstein L, Thomas PA. Adjuvant chemotherapy with cyclophosphamide, doxorubicin and cisplatin in patients with completely resected stage I non‐small‐cell lung cancer. Journal of the National Cancer Institute 1993;85(4):299‐306. - PubMed
A16 FLCSG 1 {published and unpublished data}
-
- Niiranen A, Niitamo‐Korhonen S, Kouri M, Assendelft A, Mattson K, Pyrhönen S. Adjuvant chemotherapy after radical surgery for non‐small cell lung cancer: A randomized study. Journal of Clinical Oncology 1992;10(12):1927‐32. - PubMed
A17 LCSG 853 {published and unpublished data}
-
- Figlin RA, Piantodosi S. A phase 3 randomized trial of immediate combination chemotherapy vs delayed combination chemotherapy in patients with completely resected stage II and III non‐small cell carcinoma of the lung. Chest 1994;106(Suppl 6):310S‐2S. - PubMed
A18 BLT3 {published and unpublished data}
-
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MKB, et al. Chemotherapy for patients with non‐small cell lung cancer: the surgical setting of the Big Lung Trial. European Journal of Cardio‐Thoracic Surgery 2004;26:173‐82. - PubMed
A19 SGACLC ACTLC1 {published and unpublished data}
-
- Study Group for Adjuvant Chemotherapy for Lung Cancer. A randomised controlled trial of postoperative adjuvant chemotherapy in non‐small cell lung cancer (in Japanese). Hai‐gan 1992;32:481‐6.
A20 OLCSG1c {published and unpublished data}
-
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kawahara O, Kuwabara M, et al. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166.
A21 SGACLC ACTLC2 {published and unpublished data}
-
- Study Group for Adjuvant Chemotherapy for Lung Cancer. A randomized trial of postoperative adjuvant chemotherapy in non‐small cell lung cancer (the second cooperative study). European Journal of Surgical Oncology 1995;21(1):69‐77. - PubMed
A22 WJSG 2 (1+3) {published and unpublished data}
-
- Teramatsu T, Society of adjuvant chemotherapy for lung cancer surgery in West Japan. Assessment of postoperative adjuvant chemotherapy on non‐small cell lung cancer (abstract). Lung Cancer 1991;7(Suppl):124.
-
- Wada H, Hitomi S, Takashi T, West Japan Study Group for Lung Cancer Surgery. Adjuvant chemotherapy after complete resection in non‐small cell lung cancer (full publication). Journal of Clinical Oncology 1996;14:1048‐54. - PubMed
A23 WJSG3 {published and unpublished data}
-
- Wada H, Miyahara R, Tanaka F, Hitomi S, West Japan Study Group for Lung Cancer Surgery. Post‐operative adjuvant chemotherapy with PVM (cisplatin + vindesine + mitomycin c) and UFT (uracil and tegaful) in resected stage I‐II NSCLC (non‐small cell lung cancer): a randomised clinical trial. European Journal of Cardio‐Thoracic Surgery 1999;15:438‐43. - PubMed
A24 Xu {published and unpublished data}
-
- Xu G, Rong T, Lin P. Adjuvant chemotherapy following radical surgery for non‐small cell lung cancer: a randomized study. Zhonghua Zhong Liu Za Zhi 1998;20(3):228‐30. - PubMed
A25 ACTLC4a {published and unpublished data}
-
- Imaizumi M. Postoperative adjuvant cisplatin, vindesine, plus uracil‐tegafur chemotherapy increased survival of patients with completely resected p‐stage I non‐small cell lung cancer. Lung Cancer 2005;49:85‐94. - PubMed
A26 OLCSG2b {published and unpublished data}
A27 OLCSG1b {published and unpublished data}
-
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kuwahara O, Kuwabara M, et al. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166.
A28 OLCSG1a {published and unpublished data}
-
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kuwahara O, Kuwabara M, et al. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166.
A29 WJSG2 (2+3) {published and unpublished data}
-
- Wada H, Hitomi S, Takashi T, : West Japan Study Group for Lung Cancer Surgery. Adjuvant chemotherapy after complete resection in non‐small cell lung cancer. Journal of Clinical Oncology 1996;14:1048‐54. - PubMed
A30 WJSG4 {published and unpublished data}
-
- Nakagawa M, Tanaka F, Tsubota N, Ohta M, Takao M, Wada H. A randomised phase III trial of adjuvant chemotherapy with UFT for completely resected pathological stage I non‐small cell lung cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG) ‐ the 4th study. Annals of Oncology 2005;16:75‐80. - PubMed
A31 NJSGLCS {published and unpublished data}
-
- Endo C, Saitoi Y, Iwanawi H, Tsushima T, Imai T, Kawamura M, et al. A randomized trial of postoperative UFT in p stage I, II non‐small cell lung cancer: North‐East Japan Study Group for Lung Cancer Surgery. Lung Cancer 2003;40:181‐6. - PubMed
A32 OLCSG2a {published and unpublished data}
A33 ACTLC4b {published and unpublished data}
-
- Imaizumi M. Postoperative adjuvant cisplatin, vindesine, plus uracil‐tegafur chemotherapy increased survival of patients with completely resected p‐stage I non‐small cell lung cancer. Lung Cancer 2005;49:85‐94. - PubMed
A34 JLCRG {published and unpublished data}
-
- Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomised trial of adjuvant chemotherapy with uracil‐tegafur for adenocarcinoma of the lung. New England Journal of Medicine 2004;350(17):1713‐21. - PubMed
B01 MSKCC 80‐53 {published and unpublished data}
-
- Pisters KMW, Kris MG, Gralla RT, Hilaris B, McCormack PM, Bains MS. Randomized trial comparing post‐operative chemotherapy with vindesine and cisplatin plus thoracic irradiation with irradiation alone in stage III (N2) non‐small cell lung cancer. Journal of Surgical Oncology 1994;56:236‐241. - PubMed
B02 GETCB 01CB82 {published and unpublished data}
-
- Dautzenberg B, Chastang C, Arriagada R, Chevalier T, Belpomme D, Hurdebourcq M, et al. Adjuvant radiotherapy versus combined sequential chemotherapy followed by radiotherapy in the treatment of resected non‐small cell lung cancer. Cancer 1995;76:779‐86. - PubMed
B03 EORTC 08861 {unpublished data only}
-
- EORTC Lung Cancer Cooperative Group. Phase III randomized trial of adjuvant radiotherapy vs radiotherapy plus chemotherapy with DDP/VDS vs no adjuvant therapy in patients with completely resected non‐small cell lung cancer.
B04 MDA DM 87045 {unpublished data only}
-
- MD Anderson Cancer Centre. Phase III randomized comparison of chest irradiation vs combination chemotherapy with cyclophosphamide/etoposide/cisplatine (CEP) followed by chest irradiation in patients with partially resected stage II/III limited non small cell lung cancer.
B05 INT 0115 {published and unpublished data}
-
- Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brookes BJ, et al. Postoperative adjuvant therapy in patients with stage II or IIIa non‐small cell lung cancer. New England Journal of Medicine 2000;343:1217‐22. - PubMed
B06 ALPI2 {published and unpublished data}
-
- Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II or IIIa non‐small cell lung cancer. Journal of the National Cancer Institute 2003;95(19):1453‐61. - PubMed
B07 IALT3 {published and unpublished data}
-
- Arriagada R, Dunant A, Pignon J‐P, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. Journal of Clinical Oncology 2010;28:35‐42. - PubMed
-
- The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small cell lung cancer. New England Journal of Medicine 2004;350:351‐60. - PubMed
B08 BLT4 {published and unpublished data}
-
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MKB, et al. Chemotherapy for patients with non‐small cell lung cancer: the surgical setting of the Big Lung Trial. European Journal of Cardio‐Thoracic Surgery 2004;26:173‐82. - PubMed
B09 ANITA2 {published and unpublished data}
-
- Douillard JY, Rosell R, Lena M, Carpagnano F, Ramlau R, Gonzales‐Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB‐IIIA non‐small‐cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncology 2006;7:719‐27. - PubMed
B10 IALT4 {published and unpublished data}
-
- Arriagada R, Dunant A, Pignon J‐P, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. Journal of Clinical Oncology 2010;28:35‐42. - PubMed
-
- The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small cell lung cancer. New England Journal of Medicine 2004;350:351‐60. - PubMed
B11 LCSG 791 {published and unpublished data}
-
- Lad T. The comparison of CAP chemotherapy and radiotherapy to radiotherapy alone for resected lung cancer with positive margin or involved highest sampled paratracael node (stage IIIa). Chest 1994;106(6):303S. - PubMed
-
- Lad T, Rubinstein L, Sadeghi A. The benefit of adjuvant treatment for resected locally advanced non‐small cell lung cancer. Journal of Clinical Oncology 1988;6:9‐17. - PubMed
B12 FLCSG 3 {unpublished data only}
-
- Niiranen, Kouri M, Pyrhonen S, Mattson K. Postsurgical radiotherapy versus postsurgical radiotherapy plus chemotherapy for non‐small cell lung cancer.
B13 OLCSG1d {published and unpublished data}
-
- Sawamura K, Mori T, Doi O, Yasumitsu T, Kawahara O, Kuwabara M, et al. A prospective randomized controlled study of the postoperative adjuvant therapy for non‐small cell lung cancer. Lung Cancer 1988;4:A166.
References to studies excluded from this review
Ayoub 1991 {published data only}
-
- Ayoub J, Vigneault E, Hanley J, Duranceau A, Robidoux A, Pagé A, et al. The Montreal multicenter trial in operable non‐small cell lung cancer (NSCLC): A multivariate analysis of the predictors of relapse. Proceedings of the American Society of Clinical Oncology 1991;10:247.
Clerici 1991 {published data only}
-
- Clerici M, Barni S, Cantaluppi G, et al. Adjuvant chemotherapy in non‐small cell lung cancer: a randomised trial. European Journal of Cancer 1991;27(Suppl 2):S173.
Ichinose 1991 {published data only}
-
- Ichinose Y, Hara N, Ohta M, Motohiro A, Kuda T, Aso H. Postoperative adjuvant chemotherapy in non‐small cell lung cancer: Prognostic value of DNA ploidy and postrecurrent survival. Journal of Surgical Oncology 1991;46:15‐20. - PubMed
Kim 2003 {published data only}
-
- Kim S‐W, Suh CS, Lee G‐W, Ryu M‐H, Lee SD, Kim WS, et al. The number of tumor (+) N2 nodes as a prognostic factor in the patients with N2 disease non‐small cell lung cancer after curative resection and postoperative thoracic radiotherapy. Lung Cancer 2003;41(Supplement 2):S152.
Ueda 2004 {published data only}
-
- Ueda H, Sakada T, Kuwahara M, Motohiro A. A small randomized phase III single‐center trial on postoperative UFT administration in patients with completely resected non‐small cell lung cancer. Anti‐Cancer Drugs 2004;15(1):29‐33. - PubMed
Wang 2009 {published data only}
-
- Wang S, Ou W, Sun H, Yang HX, Fang Q. Adjuvant chemotherapy in completely resected stage III‐N2 non‐small cell lung cancer. Proceedings of the American Society of Clinical Oncology 2009;27:A7563.
Wolf 2001 {published data only}
-
- Wolf M, Müller H, Seifart U, Friedel G, Hruska D, Serke M, et al. Randomized phase III trial of adjuvant radiotherapy versus adjuvant chemotherapy followed by radiotherapy in patients with N2 positive non‐small cell lung cancer (NSCLC). Proceedings of the American Society of Clinical Oncology 2001;20:311a.
Wu 2009 {published data only}
-
- Wu Y, Yang X, Chen G, Zhong W, Ben X‐S, Gu L, et al. Adjuvant docetaxel plus carboplatin compared with surgery only in patients with completely resected stage IB‐IIIA non‐small cell lung cancer: final results of CSLC201/TAX210 with Chinese Society of Lung Cancer. Journal of Thoracic Oncology 2009;4(9):S583‐S584.
Zarogoulidis 1996 {published data only}
-
- Zarogoulidis K, Filippou K, Antonio C, Papagiannis A, Hatziapostolou P, Tsiagga P, et al. The impact of adjuvant chemotherapy on the survival of postoperative stage IIIa NSCLC patients. Proceedings of the 2nd International Congress on Lung Cancer. Crete. 1996:PO35.
References to studies awaiting assessment
NATCH 2010 {published data only}
-
- Felip E, Rosell R, Maestre JA, Rodriguez‐Paniagua JM, Moran T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early‐stage non‐small‐cell lung cancer. Journal of Clinical Oncology 2010;28(19):3138‐45. - PubMed
Zheng 2011 {published data only}
-
- Zheng S, Jiang S, Li H. Adjuvant chemotherapy following radical surgery for non‐small cell lung cancer: a randomised study on 70 patients. Journal of Thoracic Oncology 2011;6(6 Suppl 2):S866.
References to ongoing studies
CALGB 30506 {unpublished data only}
-
- CALGB 30506: Phase III randomised study of adjuvant chemotherapy versus observation in patients with early stage non‐small cell lung cancer. Ongoing study March 2009.
Additional references
American Cancer Society 2007
-
- American Cancer Society. Cancer Facts and Figures 2007. www.cancer.org/acs/groups/content/@nho/documents/document/caff2007pwsecu... (accessed in December 2009) 2007.
Arriagada 2010
-
- Arriagada R, Dunant A, Pignon J‐P, Bergman B, Chabowski M, Grunenwald D, et al. Long‐term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. Journal of Clinical Oncology 2010;28(1):35‐42. - PubMed
Berghmans 2005
-
- Berghmans T, Paesmans M, Meert AP, Mascaux C, Lothaire P, Lafitte JJ, et al. Survival improvement in resectable non‐small cell lung cancer with (neo)adjuvant chemotherapy: Results of a meta‐analysis of the literature. Lung Cancer 2005;49(1):13‐23. - PubMed
Bria 2005
-
- Bria E, Gralla RJ, Raftopoulous H, Ferretti G, Felici A, Nistico C. Does adjuvant chemotherapy improve survival in non‐small cell lung cancer (NSCLC)? A pooled analysis of 6494 patients in 12 studies, examining survival and magnitude of benefit. Journal of Clinical Oncology 2005;23(16S):7140.
Butts 2010
-
- Butts CA, Ding K, Seymour L, Twumasi‐Ankrah P, Graham B, Gandara D, et al. Randomised phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage Ib and II non‐small cell lung cancer: Updated survival analysis of JBR‐10. Journal of Clinical Oncology 2010;28(1):29‐34. - PMC - PubMed
Datta 2003
-
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003;123(6):2096‐103. - PubMed
Fisher 2011
-
- Fisher DJ, Copas AJ, Tierney JF, Parmar MKB. A critical review of methods for the assessment of patient‐level interactions in individual patient data (IPD) meta‐analysis of randomised trials, and guidance for practitioners. Journal of Clinical Epidemiology 2011;64(9):949‐967. - PubMed
Hamada 2005
-
- Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, et al. Meta‐analysis of postoperative adjuvant chemotherapy with tegafur‐uracil in non‐small cell lung cancer. Journal of Clinical Oncology 2005;23(22):4999‐5006. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions ‐ Version 5.0.1 (updated in September 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hotta 2004
-
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non‐small cell lung cancer: Reappraisal with a meta‐analysis of randomised controlled trials. Journal of Clinical Oncology 2004;22(19):3860‐7. - PubMed
IASLC 2009
-
- International Association for the Study of Lung Cancer. Staging Manual in Thoracic Oncology. 1st Edition. Orange Park, FL, USA: Editorial Rx Press, 2009.
Lefebvre 2001
-
- Lefebvre C, Clarke MJ. Identifying randomised trials. In: Egger M, Smith GD, Altman DG editor(s). Systematic reviews in healthcare. 2nd Edition. London: BMJ Publishing Group, 2001:69‐87.
Lefebvre 2008
-
- Lefebvre C, Manheimer E, Glanville J, Cochrane Information Retrieval Methods Group. Searching for studies. In: Higgins JPT, Green S, Editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:95‐150.
NSCLC Collaborative Group 1995
NSCLC Collaborative Group 2000
Parkin 2005
-
- Parkin DM, Bray F, Ferlay J, Pisani Paola. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. - PubMed
Pignon 2008
-
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE Collaborative Group. Journal of Clinical Oncology 2008;26(21):3552‐9. - PubMed
Pisters 2007
-
- Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I‐IIIA resectable non small‐cell lung cancer guideline. Journal of Clinical Oncology 2007;25(34):5506‐18. - PubMed
PORT 1998
-
- PORT Meta‐analysis Trialists Group. Postoperative radiotherapy in non‐small‐cell lung cancer: systematic review and meta‐analysis of individual patient data from nine randomised controlled trials. Lancet 1998;352(9124):257‐63. - PubMed
PORT 2005
RevMan 2014 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Computer Program. Version 5.3.5 Copenhagen: the Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Schemper 1996
-
- Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Controlled Clinical Trials 1996;17(4):343‐6. - PubMed
Sedrakyan 2004
-
- Sedrakyan A, Meulen J, O'Byrne K, Prendiville J, Hill J, Treasure T. Postoperative chemotherapy for non‐small cell lung cancer: A systematic review and meta‐analysis. Journal of Thoracic and Cardiovascular Surgery 2004;128(3):414‐9. - PubMed
Sekine 2008
Toyooka 2009
-
- Toyooka S, Hotta K, Nakamura H, Nakata M, Tada H, Yamashita M, et al. A multicenter, phase III study of carboplatin/paclitaxel, versus oral uracil‐tegafur as the adjuvant chemotherapy in resected non‐small cell lung cancer (NSCLC): planned interim analyses. Proceedings of the American Society of Clinical Oncology 2009;27(15):A7560.
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

