Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome
- PMID: 25731162
- PMCID: PMC4910713
- DOI: 10.1038/nature14232
Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome
Erratum in
-
Corrigendum: Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome.Nature. 2016 Aug 11;536(7615):238. doi: 10.1038/nature18000. Epub 2016 May 4. Nature. 2016. PMID: 27144359 No abstract available.
Abstract
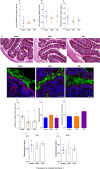
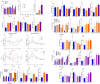
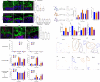
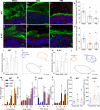
The intestinal tract is inhabited by a large and diverse community of microbes collectively referred to as the gut microbiota. While the gut microbiota provides important benefits to its host, especially in metabolism and immune development, disturbance of the microbiota-host relationship is associated with numerous chronic inflammatory diseases, including inflammatory bowel disease and the group of obesity-associated diseases collectively referred to as metabolic syndrome. A primary means by which the intestine is protected from its microbiota is via multi-layered mucus structures that cover the intestinal surface, thereby allowing the vast majority of gut bacteria to be kept at a safe distance from epithelial cells that line the intestine. Thus, agents that disrupt mucus-bacterial interactions might have the potential to promote diseases associated with gut inflammation. Consequently, it has been hypothesized that emulsifiers, detergent-like molecules that are a ubiquitous component of processed foods and that can increase bacterial translocation across epithelia in vitro, might be promoting the increase in inflammatory bowel disease observed since the mid-twentieth century. Here we report that, in mice, relatively low concentrations of two commonly used emulsifiers, namely carboxymethylcellulose and polysorbate-80, induced low-grade inflammation and obesity/metabolic syndrome in wild-type hosts and promoted robust colitis in mice predisposed to this disorder. Emulsifier-induced metabolic syndrome was associated with microbiota encroachment, altered species composition and increased pro-inflammatory potential. Use of germ-free mice and faecal transplants indicated that such changes in microbiota were necessary and sufficient for both low-grade inflammation and metabolic syndrome. These results support the emerging concept that perturbed host-microbiota interactions resulting in low-grade inflammation can promote adiposity and its associated metabolic effects. Moreover, they suggest that the broad use of emulsifying agents might be contributing to an increased societal incidence of obesity/metabolic syndrome and other chronic inflammatory diseases.
Figures







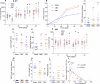
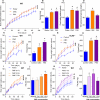

Comment in
-
Mucosal immunology: food additives feed the fire.Nat Rev Immunol. 2015 Apr;15(4):200-1. doi: 10.1038/nri3833. Epub 2015 Mar 6. Nat Rev Immunol. 2015. PMID: 25743220 No abstract available.
-
Metabolism: Dietary emulsifiers--sweepers of the gut lining?Nat Rev Endocrinol. 2015 Jun;11(6):319-20. doi: 10.1038/nrendo.2015.59. Epub 2015 Apr 14. Nat Rev Endocrinol. 2015. PMID: 25869573 No abstract available.
References
-
- Swidsinski A, Loening-Baucke V, Herber A. Mucosal flora in Crohn's disease and ulcerative colitis - an overview. J Physiol Pharmacol. 2009;60(Suppl 6):61–71. - PubMed
-
- NTP Toxicology and Carcinogenesis Studies of Polysorbate 80 (CAS No. 9005-65-6) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl Toxicol Program Tech Rep Ser. 1992;415:1–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

