Acyclic cucurbit[n]uril-type molecular containers: influence of glycoluril oligomer length on their function as solubilizing agents
- PMID: 25731639
- PMCID: PMC4366302
- DOI: 10.1039/c5ob00184f
Acyclic cucurbit[n]uril-type molecular containers: influence of glycoluril oligomer length on their function as solubilizing agents
Abstract
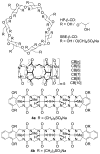
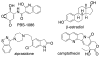
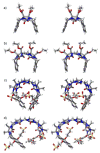
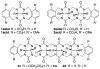
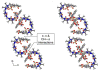
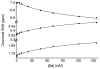
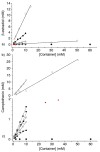
We present the synthesis of a series of six new glycoluril derived molecular clips and acyclic CB[n]-type molecular containers (1–3) that all feature SO3(−) solubilizing groups but differ in the number of glycoluril rings between the two terminal dialkoxyaromatic sidewalls. We report the X-ray crystal structure of 3b which shows that its dialkoxynaphthalene sidewalls actively define a hydrophobic cavity with high potential to engage in π–π interactions with insoluble aromatic guests. Compounds 1–3 possess very good solubility characteristics (≥38 mM) and undergo only very weak self-association (Ks < 92 M(−1)) in water. The weak self-association is attributed to unfavorable SO3(−)···SO3(−) electrostatic interactions in the putative dimers 12–42. Accordingly, we created phase solubility diagrams to study their ability to act as solubilizing agents for four water insoluble drugs (PBS-1086, camptothecin, β-estradiol, and ziprasidone). We find that the containers 3a and 3b which feature three glycoluril rings between the terminal dialkoxy-o-xylylene and dialkoxynaphthalene sidewalls are less efficient solubilizing agents than 4a and 4b because of their smaller hydrophobic cavities. Containers 1 and 2 behave as molecular clip type receptors and therefore possess the ability to bind to and thereby solubilize aromatic drugs like camptothecin, ziprasidone, and PBS-1086.
Figures


Similar articles
-
Acyclic Cucurbit[n]uril-Type Containers as Receptors for Neuromuscular Blocking Agents: Structure-Binding Affinity Relationships.Croat Chem Acta. 2019 Jul;92(2):163-171. doi: 10.5562/cca3507. Croat Chem Acta. 2019. PMID: 32855560 Free PMC article.
-
Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers.Acc Chem Res. 2014 Jul 15;47(7):2052-62. doi: 10.1021/ar500075g. Epub 2014 May 2. Acc Chem Res. 2014. PMID: 24785941 Free PMC article.
-
Uptake of Hydrocarbons in Aqueous Solution by Encapsulation in Acyclic Cucurbit[n]uril-Type Molecular Containers.Angew Chem Int Ed Engl. 2016 Jul 4;55(28):8076-80. doi: 10.1002/anie.201602671. Epub 2016 May 12. Angew Chem Int Ed Engl. 2016. PMID: 27169688
-
The cucurbit[n]uril family.Angew Chem Int Ed Engl. 2005 Aug 5;44(31):4844-70. doi: 10.1002/anie.200460675. Angew Chem Int Ed Engl. 2005. PMID: 16052668 Review.
-
Functionalized cucurbiturils and their applications.Chem Soc Rev. 2007 Feb;36(2):267-79. doi: 10.1039/b603088m. Epub 2006 Nov 7. Chem Soc Rev. 2007. PMID: 17264929 Review.
Cited by
-
Acyclic Cucurbituril Featuring Pendant Cyclodextrins.Supramol Chem. 2021;33(3):53-62. doi: 10.1080/10610278.2021.1927033. Epub 2021 May 31. Supramol Chem. 2021. PMID: 34305377 Free PMC article.
-
Glycoluril-Derived Molecular Clips are Potent and Selective Receptors for Cationic Dyes in Water.Chemistry. 2016 Oct 17;22(43):15270-15279. doi: 10.1002/chem.201601796. Epub 2016 Aug 5. Chemistry. 2016. PMID: 27492252 Free PMC article.
-
Absolute binding free energy calculations of CBClip host-guest systems in the SAMPL5 blind challenge.J Comput Aided Mol Des. 2017 Jan;31(1):71-85. doi: 10.1007/s10822-016-9968-2. Epub 2016 Sep 27. J Comput Aided Mol Des. 2017. PMID: 27677749 Free PMC article.
-
Acyclic Cucurbit[n]uril-Type Receptors: Aromatic Wall Extension Enhances Binding Affinity, Delivers Helical Chirality, and Enables Fluorescence Sensing.Chemistry. 2020 Nov 26;26(66):15249-15258. doi: 10.1002/chem.202002874. Epub 2020 Oct 16. Chemistry. 2020. PMID: 32658342 Free PMC article.
-
Blinded predictions of host-guest standard free energies of binding in the SAMPL5 challenge.J Comput Aided Mol Des. 2017 Jan;31(1):61-70. doi: 10.1007/s10822-016-9933-0. Epub 2016 Aug 8. J Comput Aided Mol Des. 2017. PMID: 27503495
References
-
- Patri AK, Kukowska-Latallo JF, Baker JR. Adv Drug Delivery Rev. 2005;57:2203–2214. - PubMed
- Blagden N, de Matas M, Gavan PT, York P. Adv Drug Delivery Rev. 2007;59:617–630. - PubMed
- Serajuddin ATM. Adv Drug Delivery Rev. 2007;59:603–616. - PubMed
- Stella VJ, Nti-Addae KW. Adv Drug Delivery Rev. 2007;59:677–694. - PubMed
-
- Freeman WA, Mock WL, Shih NY. J Am Chem Soc. 1981;103:7367–7368.
- Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K. J Am Chem Soc. 2000;122:540–541.
- Day A, Arnold AP, Blanch RJ, Snushall B. J Org Chem. 2001;66:8094–8100. - PubMed
- Liu S, Zavalij PY, Isaacs L. J Am Chem Soc. 2005;127:16798–16799. - PMC - PubMed
- Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. Angew Chem, Int Ed. 2002;41:275–277. - PubMed
- Cheng XJ, Liang LL, Chen K, Ji NN, Xiao X, Zhang JX, Zhang YQ, Xue SF, Zhu QJ, Ni XL, Tao Z. Angew Chem, Int Ed. 2013;52:7252–7255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

