Mechanobiology of the meniscus
- PMID: 25731738
- PMCID: PMC4442061
- DOI: 10.1016/j.jbiomech.2015.02.008
Mechanobiology of the meniscus
Abstract
The meniscus plays a critical biomechanical role in the knee, providing load support, joint stability, and congruity. Importantly, growing evidence indicates that the mechanobiologic response of meniscal cells plays a critical role in the physiologic, pathologic, and repair responses of the meniscus. Here we review experimental and theoretical studies that have begun to directly measure the biomechanical effects of joint loading on the meniscus under physiologic and pathologic conditions, showing that the menisci are exposed to high contact stresses, resulting in a complex and nonuniform stress-strain environment within the tissue. By combining microscale measurements of the mechanical properties of meniscal cells and their pericellular and extracellular matrix regions, theoretical and experimental models indicate that the cells in the meniscus are exposed to a complex and inhomogeneous environment of stress, strain, fluid pressure, fluid flow, and a variety of physicochemical factors. Studies across a range of culture systems from isolated cells to tissues have revealed that the biological response of meniscal cells is directly influenced by physical factors, such as tension, compression, and hydrostatic pressure. In addition, these studies have provided new insights into the mechanotransduction mechanisms by which physical signals are converted into metabolic or pro/anti-inflammatory responses. Taken together, these in vivo and in vitro studies show that mechanical factors play an important role in the health, degeneration, and regeneration of the meniscus. A more thorough understanding of the mechanobiologic responses of the meniscus will hopefully lead to therapeutic approaches to prevent degeneration and enhance repair of the meniscus.
Keywords: Articular cartilage; Collagen; Fibrochondrocyte; Mechanical signal transduction; Proteoglycan.
Copyright © 2015 Elsevier Ltd. All rights reserved.
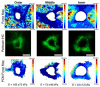
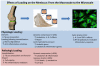
Figures




References
-
- Anderson DR, Gershuni DH, Nakhostine M, Danzig LA. The effects of non-weight-bearing and limited motion on the tensile properties of the meniscus. Arthroscopy. 1993;9:440–445. - PubMed
-
- Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: A long-term follow-up study with matched controls. Arthroscopy. 2002;18:183–189. - PubMed
-
- Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am. J. Sports Med. 1983;11:131–141. - PubMed
-
- AufderHeide AC, Athanasiou KA. Mechanical stimulation toward tissue engineering of the knee meniscus. Ann. Biomed. Eng. 2004;32:1161–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

