Dosage compensation in mammals
- PMID: 25731764
- PMCID: PMC4355265
- DOI: 10.1101/cshperspect.a019406
Dosage compensation in mammals
Abstract
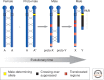
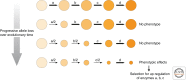
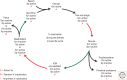
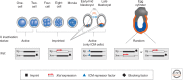
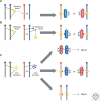
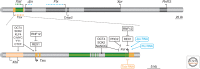
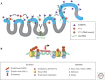
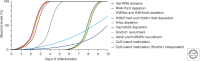
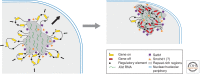
Many organisms show major chromosomal differences between sexes. In mammals, females have two copies of a large, gene-rich chromosome, the X, whereas males have one X and a small, gene-poor Y. The imbalance in expression of several hundred genes is lethal if not dealt with by dosage compensation. The male-female difference is addressed by silencing of genes on one female X early in development. However, both males and females now have only one active X chromosome. This is compensated by twofold up-regulation of genes on the active X. This complex system continues to provide important insights into mechanisms of epigenetic regulation.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Allis CD, Jenuwein T, Reinberg D 2014. Overview and concepts. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018739. - DOI
-
- Augui S, Filion GJ, Huart S, Nora E, Guggiari M, Maresca M, Stewart AF, Heard E 2007. Sensing X chromosome pairs before X inactivation via a novel X-pairing region of the Xic. Science 318: 1632–1636. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
