Targeting HIV transcription: the quest for a functional cure
- PMID: 25731772
- PMCID: PMC4409574
- DOI: 10.1007/82_2015_435
Targeting HIV transcription: the quest for a functional cure
Abstract
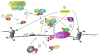
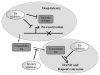
Antiretroviral therapy (ART) potently suppresses HIV-1 replication, but the virus persists in quiescent infected CD4(+)T cells as a latent integrated provirus, and patients must indefinitely remain on therapy. If ART is terminated, these integrated proviruses can reactivate, driving new rounds of infection. A functional cure for HIV requires eliminating low-level ongoing viral replication that persists in certain tissue sanctuaries and preventing viral reactivation. The HIV Tat protein plays an essential role in HIV transcription by recruiting the kinase activity of the P-TEFb complex to the viral mRNA's stem-bulge-loop structure, TAR, activating transcriptional elongation. Because the Tat-mediated transactivation cascade is critical for robust HIV replication, the Tat/TAR/P-TEFb complex is one of the most attractive targets for drug development. Importantly, compounds that interfere with transcription could impair viral reactivation, low-level ongoing replication, and replenishment of the latent reservoir, thereby reducing the size of the latent reservoir pool. Here, we discuss the potential importance of transcriptional inhibitors in the treatment of latent HIV-1 disease and review recent findings on targeting Tat, TAR, and P-TEFb individually or as part of a complex. Finally, we discuss the impact of extracellular Tat in HIV-associated neurocognitive disorders and cancers.
Figures


References
-
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2(12):1371–1375. - PubMed
-
- Aldovini A, Debouck C, Feinberg MB, Rosenberg M, Arya SK, Wong-Staal F. Synthesis of the complete trans-activation gene product of human T-lymphotropic virus type III in Escherichia coli: demonstration of immunogenicity in vivo and expression in vitro. Proc Natl Acad Sci USA. 1986;83(18):6672–6676. - PMC - PubMed
-
- Ali A, Ghosh A, Nathans RS, Sharova N, O’Brien S, Cao H, Stevenson M, Rana TM. Identification of flavopiridol analogues that selectively inhibit positive transcription elongation factor (P-TEFb) and block HIV-1 replication. Chembiochem. 2009;10(12):2072–2080. doi: 10.1002/cbic.200900303. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

