Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT
- PMID: 25732157
- PMCID: PMC4372851
- DOI: 10.1200/JCO.2014.58.2973
Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT
Erratum in
-
ERRATUM.J Clin Oncol. 2016 Jun 1;34(16):1965. doi: 10.1200/JCO.2016.68.1882. J Clin Oncol. 2016. PMID: 27217531 Free PMC article. No abstract available.
Abstract
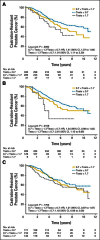
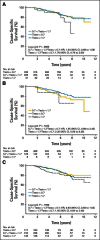
Purpose: Three small retrospective studies have suggested that patients undergoing continuous androgen deprivation (CAD) have superior survival and time to progression if lower castrate levels of testosterone (< 0.7 nmol/L) are achieved. Evidence from prospective large studies has been lacking.
Patients and methods: The PR-7 study randomly assigned patients experiencing biochemical failure after radiation therapy or surgery plus radiation therapy to CAD or intermittent androgen deprivation. The relationship between testosterone levels in the first year and cause-specific survival (CSS) and time to androgen-independent progression in men in the CAD arm was evaluated using Cox regression.
Results: There was a significant difference in CSS (P = .015) and time to hormone resistance (P = .02) among those who had first-year minimum nadir testosterone ≤ 0.7, > 0.7 to ≤ 1.7, and ≥ 1.7 nmol/L. Patients with first-year nadir testosterone consistently > 0.7 nmol/L had significantly higher risks of dying as a result of disease (0.7 to 1.7 nmol/L: hazard ratio [HR], 2.08; 95% CI, 1.28 to 3.38; > 1.7 nmol/L: HR, 2.93; 95% CI, 0.70 to 12.30) and developing hormone resistance (0.7 to 1.7 nmol/L: HR, 1.62; 95% CI, 1.20 to 2.18; ≥ 1.7 nmol/L: HR, 1.90; 95% CI, 0.77 to 4.70). Maximum testosterone ≥ 1.7 nmol/L predicted for a higher risk of dying as a result of disease (P = .02).
Conclusion: Low nadir serum testosterone (ie, < 0.7 mmol/L) within the first year of androgen-deprivation therapy correlates with improved CSS and duration of response to androgen deprivation in men being treated for biochemical failure undergoing CAD.
Trial registration: ClinicalTrials.gov NCT00003653.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures

Comment in
-
Does degree of androgen suppression matter in hormone-sensitive prostate cancer?J Clin Oncol. 2015 Apr 1;33(10):1098-100. doi: 10.1200/JCO.2014.60.1419. Epub 2015 Mar 2. J Clin Oncol. 2015. PMID: 25732171 Free PMC article. No abstract available.
-
Re: nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT.Eur Urol. 2015 Sep;68(3):537-8. doi: 10.1016/j.eururo.2015.05.027. Eur Urol. 2015. PMID: 26282354 No abstract available.
-
Re: Nadir Testosterone within First Year of Androgen-Deprivation Therapy (ADT) Predicts for Time to Castration-Resistant Progression: A Secondary Analysis of the PR-7 Trial of Intermittent versus Continuous ADT.J Urol. 2016 Jun;195(6):1779-82. doi: 10.1016/j.juro.2016.03.056. Epub 2016 Mar 17. J Urol. 2016. PMID: 27191068 No abstract available.
Similar articles
-
Re: nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT.Eur Urol. 2015 Sep;68(3):537-8. doi: 10.1016/j.eururo.2015.05.027. Eur Urol. 2015. PMID: 26282354 No abstract available.
-
Interpreting Testosterone and Concomitant Prostate Specific Antigen Values during Androgen Deprivation Therapy for Recurrent Prostate Cancer.J Urol. 2021 Nov;206(5):1166-1176. doi: 10.1097/JU.0000000000001946. Epub 2021 Jun 29. J Urol. 2021. PMID: 34184929 Clinical Trial.
-
Testosterone Responders to Continuous Androgen Deprivation Therapy Show Considerable Variations in Testosterone Levels on Followup: Implications for Clinical Practice.J Urol. 2018 Jan;199(1):251-256. doi: 10.1016/j.juro.2017.07.078. Epub 2017 Jul 25. J Urol. 2018. PMID: 28751266
-
Testosterone Levels and Prostate Cancer Prognosis: Systematic Review and Meta-analysis.Clin Genitourin Cancer. 2018 Jun;16(3):165-175.e2. doi: 10.1016/j.clgc.2018.01.005. Epub 2018 Feb 2. Clin Genitourin Cancer. 2018. PMID: 29454638
-
Intermittent androgen-deprivation therapy in prostate cancer: a critical review focused on phase 3 trials.Eur Urol. 2013 Nov;64(5):722-30. doi: 10.1016/j.eururo.2013.04.020. Epub 2013 Apr 19. Eur Urol. 2013. PMID: 23628492 Review.
Cited by
-
Pharmacokinetic and pharmacodynamic comparison of subcutaneous versus intramuscular leuprolide acetate formulations in male subjects.Ther Adv Urol. 2017 Nov 22;10(2):43-50. doi: 10.1177/1756287217738150. eCollection 2018 Feb. Ther Adv Urol. 2017. PMID: 29434672 Free PMC article.
-
The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism.Prostate Cancer Prostatic Dis. 2016 Jun;19(2):191-6. doi: 10.1038/pcan.2016.2. Epub 2016 Feb 9. Prostate Cancer Prostatic Dis. 2016. PMID: 26857022
-
Pericytes in castration-resistant prostate cancer associated with disease progression and immunotherapy response: insights from single-cell analysis.Cancer Cell Int. 2025 Jun 3;25(1):200. doi: 10.1186/s12935-025-03838-3. Cancer Cell Int. 2025. PMID: 40462105 Free PMC article.
-
Review of the Reporting of Survival Analyses within Randomised Controlled Trials and the Implications for Meta-Analysis.PLoS One. 2016 May 5;11(5):e0154870. doi: 10.1371/journal.pone.0154870. eCollection 2016. PLoS One. 2016. PMID: 27149107 Free PMC article. Review.
-
Sex steroid modulation of macrophages within the prostate tumor microenvironment.Am J Clin Exp Urol. 2022 Apr 15;10(2):98-110. eCollection 2022. Am J Clin Exp Urol. 2022. PMID: 35528461 Free PMC article.
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate—1941. J Urol. 2002;168:9–12. - PubMed
-
- Morote J, Planas J, Salvador C, et al. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2009;103:332–335. - PubMed
-
- Pickles T, Hamm J, Morris WJ, et al. Incomplete testosterone suppression with luteinizing hormone-releasing hormone agonists: Does it happen and does it matter? BJU Int. 2012;110:E500–E507. - PubMed
-
- Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–1295. - PubMed
-
- Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: Prognostic significance? BJU Int. 2010;105:648–645. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

