Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance)
- PMID: 25732163
- PMCID: PMC4429175
- DOI: 10.1200/JCO.2014.57.3105
Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance)
Abstract
Purpose: To evaluate the efficacy of maintenance sunitinib after chemotherapy for small-cell lung cancer (SCLC).
Patients and methods: The Cancer and Leukemia Group B 30504 trial was a randomized, placebo-controlled, phase II study that enrolled patients before chemotherapy (cisplatin 80 mg/m(2) or carboplatin area under the curve of 5 on day 1 plus etoposide 100 mg/m(2) per day on days 1 to 3 every 21 days for four to six cycles). Patients without progression were randomly assigned 1:1 to placebo or sunitinib 37.5 mg per day until progression. Cross-over after progression was allowed. The primary end point was progression-free survival (PFS) from random assignment for maintenance placebo versus sunitinib using a one-sided log-rank test with α = .15; 80 randomly assigned patients provided 89% power to detect a hazard ratio (HR) of 1.67.
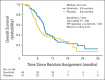
Results: One hundred forty-four patients were enrolled; 138 patients received chemotherapy. Ninety-five patients were randomly assigned; 10 patients did not receive maintenance therapy (five on each arm). Eighty-five patients received maintenance therapy (placebo, n = 41; sunitinib, n = 44). Grade 3 adverse events with more than 5% incidence were fatigue (19%), decreased neutrophils (14%), decreased leukocytes (7%), and decreased platelets (7%) for sunitinib and fatigue (10%) for placebo; grade 4 adverse events were GI hemorrhage (n = 1) and pancreatitis, hypocalcemia, and elevated lipase (n = 1; all in same patient) for sunitinib and thrombocytopenia (n = 1) and hypernatremia (n = 1) for placebo. Median PFS on maintenance was 2.1 months for placebo and 3.7 months for sunitinib (HR, 1.62; 70% CI, 1.27 to 2.08; 95% CI, 1.02 to 2.60; one-sided P = .02). Median overall survival from random assignment was 6.9 months for placebo and 9.0 months for sunitinib (HR, 1.28; 95% CI, 0.79 to 2.10; one-sided P = .16). Three sunitinib and no placebo patients achieved complete response during maintenance. Ten (77%) of 13 patients evaluable after cross-over had stable disease on sunitinib (6 to 27 weeks).
Conclusion: Maintenance sunitinib was safe and improved PFS in extensive-stage SCLC.
Trial registration: ClinicalTrials.gov NCT00453154.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



Comment in
-
Sunitinib: the next advance in small-cell lung cancer?J Clin Oncol. 2015 May 20;33(15):1637-9. doi: 10.1200/JCO.2014.60.1815. Epub 2015 Apr 13. J Clin Oncol. 2015. PMID: 25870089 No abstract available.
References
-
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(suppl 1):191–202. - PubMed
-
- Miller AA, Herndon JE, 2nd, Hollis DR, et al. Schedule dependency of 21 day oral versus 3 day IV etoposide in combination with IV cisplatin in extensive stage small cell lung cancer: A randomized phase III study of the Cancer and Leukemia Group B study group. J Clin Oncol. 1995;13:1871–1879. - PubMed
-
- Niell HB, Herndon JE, 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23:3752–3759. - PubMed
-
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

