Nomograms Predicting Progression-Free Survival, Overall Survival, and Pelvic Recurrence in Locally Advanced Cervical Cancer Developed From an Analysis of Identifiable Prognostic Factors in Patients From NRG Oncology/Gynecologic Oncology Group Randomized Trials of Chemoradiotherapy
- PMID: 25732170
- PMCID: PMC4477785
- DOI: 10.1200/JCO.2014.57.7122
Nomograms Predicting Progression-Free Survival, Overall Survival, and Pelvic Recurrence in Locally Advanced Cervical Cancer Developed From an Analysis of Identifiable Prognostic Factors in Patients From NRG Oncology/Gynecologic Oncology Group Randomized Trials of Chemoradiotherapy
Abstract
Purpose: To evaluate the prognostic factors in locally advanced cervical cancer limited to the pelvis and develop nomograms for 2-year progression-free survival (PFS), 5-year overall survival (OS), and pelvic recurrence.
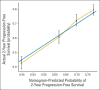
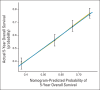
Patients and methods: We retrospectively reviewed 2,042 patients with locally advanced cervical carcinoma enrolled onto Gynecologic Oncology Group clinical trials of concurrent cisplatin-based chemotherapy and radiotherapy. Nomograms for 2-year PFS, five-year OS, and pelvic recurrence were created as visualizations of Cox proportional hazards regression models. The models were validated by bootstrap-corrected, relatively unbiased estimates of discrimination and calibration.
Results: Multivariable analysis identified prognostic factors including histology, race/ethnicity, performance status, tumor size, International Federation of Gynecology and Obstetrics stage, tumor grade, pelvic node status, and treatment with concurrent cisplatin-based chemotherapy. PFS, OS, and pelvic recurrence nomograms had bootstrap-corrected concordance indices of 0.62, 0.64, and 0.73, respectively, and were well calibrated.
Conclusion: Prognostic factors were used to develop nomograms for 2-year PFS, 5-year OS, and pelvic recurrence for locally advanced cervical cancer clinically limited to the pelvis treated with concurrent cisplatin-based chemotherapy and radiotherapy. These nomograms can be used to better estimate individual and collective outcomes.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



References
-
- Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. - PubMed
-
- Stehman FB, Bundy BN, DiSaia PJ, et al. Carcinoma of the cervix treated with radiation therapy: I. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–2785. - PubMed
-
- National Institutes of Health. NCI issues clinical announcement on cervical cancer: Chemotherapy plus radiation improves survival. http://www.nih.gov/news/pr/feb99/nci-22.htm.
-
- Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. - PubMed
-
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. - PubMed

