Paracetamol: not as safe as we thought? A systematic literature review of observational studies
- PMID: 25732175
- PMCID: PMC4789700
- DOI: 10.1136/annrheumdis-2014-206914
Paracetamol: not as safe as we thought? A systematic literature review of observational studies
Abstract
Objectives: We conducted a systematic literature review to assess the adverse event (AE) profile of paracetamol.
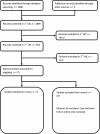
Methods: We searched Medline and Embase from database inception to 1 May 2013. We screened for observational studies in English, which reported mortality, cardiovascular, gastrointestinal (GI) or renal AEs in the general adult population at standard analgesic doses of paracetamol. Study quality was assessed using Grading of Recommendations Assessment, Development and Evaluation. Pooled or adjusted summary statistics were presented for each outcome.
Results: Of 1888 studies retrieved, 8 met inclusion criteria, and all were cohort studies. Comparing paracetamol use versus no use, of two studies reporting mortality one showed a dose-response and reported an increased relative rate of mortality from 0.95 (0.92 to 0.98) to 1.63 (1.58 to 1.68). Of four studies reporting cardiovascular AEs, all showed a dose-response with one reporting an increased risk ratio of all cardiovascular AEs from 1.19 (0.81 to 1.75) to 1.68 (1.10 to 2.57). One study reporting GI AEs reported a dose-response with increased relative rate of GI AEs or bleeds from 1.11 (1.04 to 1.18) to 1.49 (1.34 to 1.66). Of four studies reporting renal AEs, three reported a dose-response with one reporting an increasing OR of ≥30% decrease in estimated glomerular filtration rate from 1.40 (0.79 to 2.48) to 2.19 (1.4 to 3.43).
Discussion: Given the observational nature of the data, channelling bias may have had an important impact. However, the dose-response seen for most endpoints suggests a considerable degree of paracetamol toxicity especially at the upper end of standard analgesic doses.
Keywords: Epidemiology; Osteoarthritis; Outcomes research.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures




Comment in
-
Did the subjects and the controls have the same disease?Ann Rheum Dis. 2016 Jul;75(7):e43. doi: 10.1136/annrheumdis-2015-207692. Epub 2015 Apr 20. Ann Rheum Dis. 2016. PMID: 25897017 No abstract available.
-
Paracetamol: a probably still safe drug.Ann Rheum Dis. 2016 Sep;75(9):e57. doi: 10.1136/annrheumdis-2016-209713. Epub 2016 May 10. Ann Rheum Dis. 2016. PMID: 27165178 No abstract available.
-
COVID-19: thoughts at sunrise.Intern Emerg Med. 2020 Nov;15(8):1579-1580. doi: 10.1007/s11739-020-02344-w. Epub 2020 May 9. Intern Emerg Med. 2020. PMID: 32388835 Free PMC article. No abstract available.
References
-
- Jordan KM, Arden NK, Doherty M, et al. . EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145–55. 10.1136/ard.2003.011742 - DOI - PMC - PubMed
-
- Zhang W, Nuki G, Moskowitz RW, et al. . OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–99. 10.1016/j.joca.2010.01.013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

