Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer's disease
- PMID: 25732182
- PMCID: PMC4542619
- DOI: 10.1093/brain/awv024
Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer's disease
Abstract
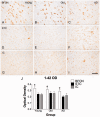
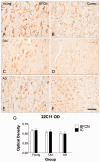
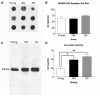
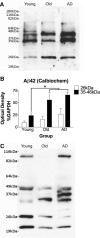
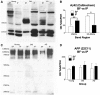
The mechanisms that contribute to selective vulnerability of the magnocellular basal forebrain cholinergic neurons in neurodegenerative diseases, such as Alzheimer's disease, are not fully understood. Because age is the primary risk factor for Alzheimer's disease, mechanisms of interest must include age-related alterations in protein expression, cell type-specific markers and pathology. The present study explored the extent and characteristics of intraneuronal amyloid-β accumulation, particularly of the fibrillogenic 42-amino acid isoform, within basal forebrain cholinergic neurons in normal young, normal aged and Alzheimer's disease brains as a potential contributor to the selective vulnerability of these neurons using immunohistochemistry and western blot analysis. Amyloid-β1-42 immunoreactivity was observed in the entire cholinergic neuronal population regardless of age or Alzheimer's disease diagnosis. The magnitude of this accumulation as revealed by optical density measures was significantly greater than that in cortical pyramidal neurons, and magnocellular neurons in the globus pallidus did not demonstrate a similar extent of amyloid immunoreactivity. Immunoblot analysis with a panel of amyloid-β antibodies confirmed accumulation of high concentration of amyloid-β in basal forebrain early in adult life. There was no age- or Alzheimer-related alteration in total amyloid-β content within this region. In contrast, an increase in the large molecular weight soluble oligomer species was observed with a highly oligomer-specific antibody in aged and Alzheimer brains when compared with the young. Similarly, intermediate molecular weight oligomeric species displayed an increase in aged and Alzheimer brains when compared with the young using two amyloid-β42 antibodies. Compared to cortical homogenates, small molecular weight oligomeric species were lower and intermediate species were enriched in basal forebrain in ageing and Alzheimer's disease. Regional and age-related differences in accumulation were not the result of alterations in expression of the amyloid precursor protein, as confirmed by both immunostaining and western blot. Our results demonstrate that intraneuronal amyloid-β accumulation is a relatively selective trait of basal forebrain cholinergic neurons early in adult life, and increases in the prevalence of intermediate and large oligomeric assembly states are associated with both ageing and Alzheimer's disease. Selective intraneuronal amyloid-β accumulation in adult life and oligomerization during the ageing process are potential contributors to the degeneration of basal forebrain cholinergic neurons in Alzheimer's disease.
Keywords: Alzheimer pathology; amyloid oligomer; amyloid-β; basal forebrain cholinergic neurons; intracellular.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Intraneuronal amyloid-β accumulation in basal forebrain cholinergic neurons: a marker of vulnerability, yet inversely related to neurodegeneration.Brain. 2015 Jun;138(Pt 6):1444-5. doi: 10.1093/brain/awv097. Brain. 2015. PMID: 26013803 Free PMC article.
References
-
- Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, et al. Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Res Rev. 2003;43:1–16. - PubMed
-
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid β protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J Comp Neurol. 1998;397:139–47. - PubMed
-
- Bao F, Wicklund L, Lacor PN, Klein WL, Nordberg A, Marutle A. Different β-amyloid oligomer assemblies in alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging. 2012;33:825–e1–13. - PubMed
-
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, et al. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–60. - PubMed
-
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

