Epithelial NAIPs protect against colonic tumorigenesis
- PMID: 25732303
- PMCID: PMC4354369
- DOI: 10.1084/jem.20140474
Epithelial NAIPs protect against colonic tumorigenesis
Abstract
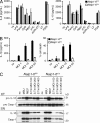
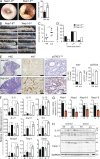
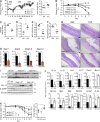
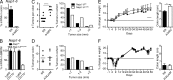
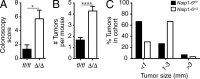
NLR family apoptosis inhibitory proteins (NAIPs) belong to both the Nod-like receptor (NLR) and the inhibitor of apoptosis (IAP) families. NAIPs are known to form an inflammasome with NLRC4, but other in vivo functions remain unexplored. Using mice deficient for all NAIP paralogs (Naip1-6(Δ/Δ)), we show that NAIPs are key regulators of colorectal tumorigenesis. Naip1-6(Δ/Δ) mice developed increased colorectal tumors, in an epithelial-intrinsic manner, in a model of colitis-associated cancer. Increased tumorigenesis, however, was not driven by an exacerbated inflammatory response. Instead, Naip1-6(Δ/Δ) mice were protected from severe colitis and displayed increased antiapoptotic and proliferation-related gene expression. Naip1-6(Δ/Δ) mice also displayed increased tumorigenesis in an inflammation-independent model of colorectal cancer. Moreover, Naip1-6(Δ/Δ) mice, but not Nlrc4-null mice, displayed hyper-activation of STAT3 and failed to activate p53 18 h after carcinogen exposure. This suggests that NAIPs protect against tumor initiation in the colon by promoting the removal of carcinogen-elicited epithelium, likely in a NLRC4 inflammasome-independent manner. Collectively, we demonstrate a novel epithelial-intrinsic function of NAIPs in protecting the colonic epithelium against tumorigenesis.
© 2015 Allam et al.
Figures

References
-
- Allen I.C., TeKippe E.M., Woodford R.M., Uronis J.M., Holl E.K., Rogers A.B., Herfarth H.H., Jobin C., and Ting J.P.. 2010. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207:1045–1056 10.1084/jem.20100050 - DOI - PMC - PubMed
-
- Bollrath J., Phesse T.J., von Burstin V.A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., et al. . 2009. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 15:91–102 10.1016/j.ccr.2009.01.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

