Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines
- PMID: 25732306
- PMCID: PMC4354366
- DOI: 10.1084/jem.20141702
Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines
Abstract
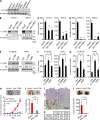
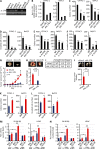
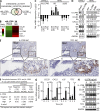
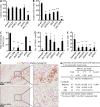
Pancreatic cancer has an extremely high mortality rate due to its aggressive metastatic nature. Resolving the underlying mechanisms will be crucial for treatment. Here, we found that overexpression of IL-17B receptor (IL-17RB) strongly correlated with postoperative metastasis and inversely correlated with progression-free survival in pancreatic cancer patients. Consistently, results from ex vivo experiments further validated that IL-17RB and its ligand, IL-17B, plays an essential role in pancreatic cancer metastasis and malignancy. Signals from IL-17B-IL-17RB activated CCL20/CXCL1/IL-8/TFF1 chemokine expressions via the ERK1/2 pathway to promote cancer cell invasion, macrophage and endothelial cell recruitment at primary sites, and cancer cell survival at distant organs. Treatment with a newly derived monoclonal antibody against IL-17RB blocked tumor metastasis and promoted survival in a mouse xenograft model. These findings not only illustrate a key mechanism underlying the highly aggressive characteristics of pancreatic cancer but also provide a practical approach to tackle this disease.
© 2015 Wu et al.
Figures



Comment in
-
Interleukin interrupted: a new strategy for the treatment of pancreatic cancer.J Exp Med. 2015 Mar 9;212(3):284. doi: 10.1084/jem.2123insight1. J Exp Med. 2015. PMID: 25754118 Free PMC article. No abstract available.
Similar articles
-
The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer.Front Immunol. 2020 Apr 21;11:718. doi: 10.3389/fimmu.2020.00718. eCollection 2020. Front Immunol. 2020. PMID: 32373132 Free PMC article. Review.
-
Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway.Oncogene. 2014 Jun 5;33(23):2968-77. doi: 10.1038/onc.2013.268. Epub 2013 Jul 15. Oncogene. 2014. PMID: 23851503
-
Non-tumor tissue derived interleukin-17B activates IL-17RB/AKT/β-catenin pathway to enhance the stemness of gastric cancer.Sci Rep. 2016 May 5;6:25447. doi: 10.1038/srep25447. Sci Rep. 2016. PMID: 27146881 Free PMC article.
-
Differential roles of IL-17B and IL-17RB in colorectal cancer: Correlation with immune infiltration and prognosis.Pathol Res Pract. 2025 Apr;268:155847. doi: 10.1016/j.prp.2025.155847. Epub 2025 Feb 24. Pathol Res Pract. 2025. PMID: 40020328
-
IL-17B: A new area of study in the IL-17 family.Mol Immunol. 2017 Oct;90:50-56. doi: 10.1016/j.molimm.2017.07.004. Epub 2017 Jul 10. Mol Immunol. 2017. PMID: 28704706 Review.
Cited by
-
The Role of Airway Epithelial Cell Alarmins in Asthma.Cells. 2022 Mar 24;11(7):1105. doi: 10.3390/cells11071105. Cells. 2022. PMID: 35406669 Free PMC article. Review.
-
CD4+ T cells are required to improve the efficacy of CIK therapy in non-small cell lung cancer.Cell Death Dis. 2022 May 6;13(5):441. doi: 10.1038/s41419-022-04882-x. Cell Death Dis. 2022. PMID: 35523765 Free PMC article. Clinical Trial.
-
Inflammatory Cytokine Signaling during Development of Pancreatic and Prostate Cancers.J Immunol Res. 2017;2017:7979637. doi: 10.1155/2017/7979637. Epub 2017 Dec 12. J Immunol Res. 2017. PMID: 29379802 Free PMC article. Review.
-
T Lymphocytes: A Promising Immunotherapeutic Target for Pancreatitis and Pancreatic Cancer?Front Oncol. 2020 Mar 24;10:382. doi: 10.3389/fonc.2020.00382. eCollection 2020. Front Oncol. 2020. PMID: 32266154 Free PMC article. Review.
-
The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer.Front Immunol. 2020 Apr 21;11:718. doi: 10.3389/fimmu.2020.00718. eCollection 2020. Front Immunol. 2020. PMID: 32373132 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

