Futile cycling increases sensitivity toward oxidative stress in Escherichia coli
- PMID: 25732623
- PMCID: PMC4430396
- DOI: 10.1016/j.ymben.2015.02.006
Futile cycling increases sensitivity toward oxidative stress in Escherichia coli
Abstract
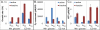
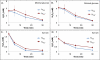
Reactive oxygen species (ROS) are toxic molecules utilized by the immune system to combat invading pathogens. Recent evidence suggests that inefficiencies in ATP production or usage can lead to increased endogenous ROS production and sensitivity to oxidative stress in bacteria. With this as inspiration, and knowledge that ATP is required for a number of DNA repair mechanisms, we hypothesized that futile cycling would be an effective way to increase sensitivity to oxidative stress. We developed a mixed integer linear optimization framework to identify experimentally-tractable futile cycles, and confirmed metabolic modeling predictions that futile cycling depresses growth rate, and increases both O2 consumption and ROS production per biomass generated. Further, intracellular ATP was decreased and sensitivity to oxidative stress increased in all actively cycling strains compared to their catalytically inactive controls. This research establishes a fundamental connection between ATP metabolism, endogenous ROS production, and tolerance toward oxidative stress in bacteria.
Keywords: Futile cycle; Hydrogen peroxide; Metabolism; Oxidative stress; Reactive oxygen species.
Copyright © 2015 International Metabolic Engineering Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Ahmad Z, Senior AE. Mutagenesis of residue betaArg-246 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F1-ATPase. J Biol Chem. 2004;279:31505–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

