Caspase-mediated apoptosis in the cochleae contributes to the early onset of hearing loss in A/J mice
- PMID: 25732708
- PMCID: PMC4366423
- DOI: 10.1177/1759091415573985
Caspase-mediated apoptosis in the cochleae contributes to the early onset of hearing loss in A/J mice
Abstract
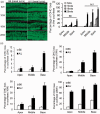
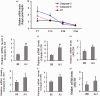
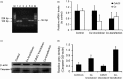
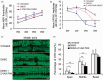
A/J and C57BL/6 J (B6) mice share a mutation in Cdh23 (ahl allele) and are characterized by age-related hearing loss. However, hearing loss occurs much earlier in A/J mice at about four weeks of age. Recent study has revealed that a mutation in citrate synthase (Cs) is one of the main contributors, but the mechanism is largely unknown. In the present study, we showed that A/J mice displayed more severe degeneration of hair cells, spiral ganglion neurons, and stria vascularis in the cochleae compared with B6 mice. Moreover, messenger RNA accumulation levels of caspase-3 and caspase-9 in the inner ears of A/J mice were significantly higher than those in B6 mice at 2 and 8 weeks of age. Immunohistochemistry localized caspase-3 expression mainly to the hair cells, spiral ganglion neurons, and stria vascularis in cochleae. In vitro transfection with Cs short hairpin RNA (shRNA) alone or cotransfection with Cs shRNA and Cdh23 shRNA significantly increased the levels of caspase-3 in an inner ear cell line (HEI-OC1). Finally, a pan-caspase inhibitor Z-VAD-FMK could preserve the hearing of A/J mice by lowering about 15 decibels of the sound pressure level for the auditory-evoked brainstem response thresholds. In conclusion, our results suggest that caspase-mediated apoptosis in the cochleae, which may be related to a Cs mutation, contributes to the early onset of hearing loss in A/J mice.
Figures


References
-
- Alam S. A., Oshima T., Suzuki M., Kawase T., Takasaka T., Ikeda K. (2001) The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope 111: 528–534. - PubMed
-
- Balaban R. S., Nemoto S., Finkel T. (2005) Mitochondria, oxidants, and aging. Cell 120: 483–495. - PubMed
-
- Cheng T. L., Liao C. C., Tsai W. H., Lin C. C., Yeh C. W., Teng C. F., Chang W. T. (2009) Identification and characterization of the mitochondrial targeting sequence and mechanism in human citrate synthase. Journal of Cellular Biochemistry 107: 1002–1015. - PubMed
-
- Dirks A. J., Hofer T., Marzetti E., Pahor M., Leeuwenburgh C. (2006) Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Research Reviews 5: 179–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

