New tools for the quantitative assessment of prodrug delivery and neurotoxicity
- PMID: 25732874
- PMCID: PMC4501381
- DOI: 10.1016/j.neuro.2015.02.005
New tools for the quantitative assessment of prodrug delivery and neurotoxicity
Abstract
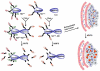
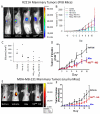
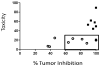
Systemic off-target toxicities, including neurotoxicity, are prevalent side effects in cancer patients treated with a number of otherwise highly efficacious anticancer drugs. In the current study, we have: (1) developed a new analytical metric for the in vivo preclinical assessment of systemic toxicities/neurotoxicity of new drugs and delivery systems; and (2) evaluated, in mice, the in vivo efficacy and toxicity of a versatile and modular NanoDendron (ND) drug delivery and imaging platform that we recently developed. Our paclitaxel-carrying ND prodrug, ND(PXL), is activated following proteolytic cleavage by MMP9, resulting in localized cytotoxic chemotherapy. Using click chemistry, we combined ND(PXL) with a traceable beacon, ND(PB), yielding ND(PXL)-ND(PB) that functions as a theranostic compound. In vivo fluorescence FRET imaging of this theranostic platform was used to confirm localized delivery to tumors and to assess the efficiency of drug delivery to tumors, achieving 25-30% activation in the tumors of an immunocompetent mouse model of breast cancer. In this model, ND-drug exhibited anti-tumor efficacy comparable to nab-paclitaxel, a clinical formulation. In addition, we combined neurobehavioral metrics of nociception and sensorimotor performance of individual mice to develop a novel composite toxicity score that reveals and quantifies peripheral neurotoxicity, a debilitating long-term systemic toxicity of paclitaxel therapy. Importantly, mice treated with nab-paclitaxel developed changes in behavioral metrics with significantly higher toxicity scores indicative of peripheral neuropathy, while mice treated with ND(PXL) showed no significant changes in behavioral responses or toxicity score. Our ND formulation was designed to be readily adaptable to incorporate different drugs, imaging modalities and/or targeting motifs. This formulation has significant potential for preclinical and clinical tools across multiple disease states. The studies presented here report a novel toxicity score for assessing peripheral neuropathy and demonstrate that our targeted, theranostic NDs are safe and effective, providing localized tumor delivery of a chemotherapeutic and with reduced common neurotoxic side-effects.
Keywords: Neuropathy; Protease-mediated drug delivery; Protease-mediated imaging; Theranostic paclitaxel; Toxicity assessment.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

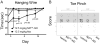


References
-
- Achilefu S. “Rapid response activatable molecular probes for intraoperative optical image-guided tumor resection. Hepatology. 2012;56(3):1170–1173. - PubMed
-
- Ahmed N, Fessi H, Elaissari A. “Theranostic applications of nanoparticles in cancer. Drug Discov Today. 2012;17(17-18):928–934. - PubMed
-
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. “Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82(1):51–77. - PubMed
-
- Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. “Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66(3):218–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

