Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs
- PMID: 25733017
- PMCID: PMC4375934
- DOI: 10.1016/j.stemcr.2015.01.016
Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs
Abstract
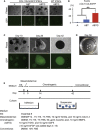
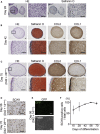
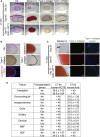
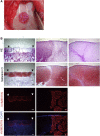
Defects in articular cartilage ultimately result in loss of joint function. Repairing cartilage defects requires cell sources. We developed an approach to generate scaffoldless hyaline cartilage from human induced pluripotent stem cells (hiPSCs). We initially generated an hiPSC line that specifically expressed GFP in cartilage when teratoma was formed. We optimized the culture conditions and found BMP2, transforming growth factor β1 (TGF-β1), and GDF5 critical for GFP expression and thus chondrogenic differentiation of the hiPSCs. The subsequent use of scaffoldless suspension culture contributed to purification, producing homogenous cartilaginous particles. Subcutaneous transplantation of the hiPSC-derived particles generated hyaline cartilage that expressed type II collagen, but not type I collagen, in immunodeficiency mice. Transplantation of the particles into joint surface defects in immunodeficiency rats and immunosuppressed mini-pigs indicated that neocartilage survived and had potential for integration into native cartilage. The immunodeficiency mice and rats suffered from neither tumors nor ectopic tissue formation. The hiPSC-derived cartilaginous particles constitute a viable cell source for regenerating cartilage defects.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Bai H.Y., Chen G.A., Mao G.H., Song T.R., Wang Y.X. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J. Biomed. Mater. Res. A. 2010;94:539–546. - PubMed
-
- Bigdeli N., Karlsson C., Strehl R., Concaro S., Hyllner J., Lindahl A. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells. 2009;27:1812–1821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

