Nuclear signaling from cadherin adhesion complexes
- PMID: 25733140
- PMCID: PMC4410048
- DOI: 10.1016/bs.ctdb.2014.11.018
Nuclear signaling from cadherin adhesion complexes
Abstract
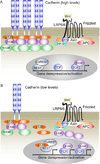
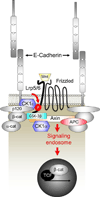
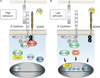
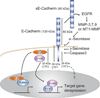
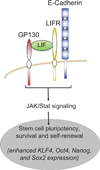
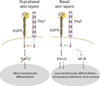
The arrival of multicellularity in evolution facilitated cell-cell signaling in conjunction with adhesion. As the ectodomains of cadherins interact with each other directly in trans (as well as in cis), spanning the plasma membrane and associating with multiple other entities, cadherins enable the transduction of "outside-in" or "inside-out" signals. We focus this review on signals that originate from the larger family of cadherins that are inwardly directed to the nucleus, and thus have roles in gene control or nuclear structure-function. The nature of cadherin complexes varies considerably depending on the type of cadherin and its context, and we will address some of these variables for classical cadherins versus other family members. Substantial but still fragmentary progress has been made in understanding the signaling mediators used by varied cadherin complexes to coordinate the state of cell-cell adhesion with gene expression. Evidence that cadherin intracellular binding partners also localize to the nucleus is a major point of interest. In some models, catenins show reduced binding to cadherin cytoplasmic tails favoring their engagement in gene control. When bound, cadherins may serve as stoichiometric competitors of nuclear signals. Cadherins also directly or indirectly affect numerous signaling pathways (e.g., Wnt, receptor tyrosine kinase, Hippo, NFκB, and JAK/STAT), enabling cell-cell contacts to touch upon multiple biological outcomes in embryonic development and tissue homeostasis.
Keywords: Atypical cadherins; Desmosomal cadherins; Hippo signaling; Outside-in signaling; Protocadherins; RTK signaling; Wnt signaling.
© 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. - PubMed
-
- Aktary Z, Kulak S, Mackey J, Jahroudi N, Pasdar M. Plakoglobin interacts with the transcription factor p53 and regulates the expression of 14-3-3 sigma. Journal of Cell Science. 2013;126:3031–3042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

