Transcriptional regulation of renal dopamine D1 receptor function during oxidative stress
- PMID: 25733244
- PMCID: PMC4393374
- DOI: 10.1161/HYPERTENSIONAHA.115.05255
Transcriptional regulation of renal dopamine D1 receptor function during oxidative stress
Abstract
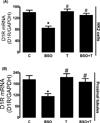
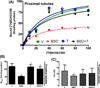
There exists a strong link between oxidative stress, renal dopaminergic system, and hypertension. It is reported that reactive oxygen species attenuate renal proximal tubular dopamine receptor (D1R) function, which disrupts sodium regulation and leads to hypertension. However, the mechanisms for renal D1R dysfunction are not clear. We investigated the role of redox-sensitive transcription factors AP1 and SP3 in transcriptional suppression of D1R gene and subsequent D1R signaling. Human kidney proximal tubular cells were treated with a pro-oxidant l-buthionine sulfoximine (BSO) with and without an antioxidant tempol. In human kidney cells, BSO caused oxidative stress and reduced D1R mRNA and membrane receptor expression. Incubation of human kidney cells with SKF38393, a D1R agonist, caused a concentration-dependent inhibition of Na/K-ATPase. However, SKF38393 failed to inhibit Na/K-ATPase in BSO-treated cells. BSO increased AP1 and SP3 nuclear expression. Transfection with AP1- or SP3-specific siRNA abolished BSO-induced D1R downregulation. Treatment of rats with BSO for 4 weeks increased oxidative stress and SP3-AP1 expression and reduced D1R numbers in renal proximal tubules. These rats exhibited high blood pressure, and SKF38393 failed to inhibit proximal tubular Na/K-ATPase activity. Control rats were kept on tap water. Tempol per se had no effect on D1R expression or other signaling molecules but prevented BSO-induced oxidative stress, SP3-AP1 upregulation, and D1R dysfunction in both human kidney cells and rats. These data show that oxidative stress via AP1-SP3 activation suppresses D1R transcription and function. Tempol mitigates oxidative stress, blocks AP1-SP3 activation, and prevents D1R dysfunction and hypertension.
Keywords: GTP-binding proteins; antioxidants; hypertension; kidney; kidney tubules, proximal.
© 2015 American Heart Association, Inc.
Conflict of interest statement
None
Figures




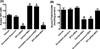



Comment in
-
Hypertension: Redox-sensitive transcription factors regulate the renal dopamine receptor.Nat Rev Nephrol. 2015 May;11(5):255. doi: 10.1038/nrneph.2015.32. Epub 2015 Mar 17. Nat Rev Nephrol. 2015. PMID: 25776561 No abstract available.
References
-
- Guyton AC. Dominant role of the kidneys and accessory role of whole-body autoregulation in the pathogenesis of hypertension. American journal of hypertension. 1989;2:575–585. - PubMed
-
- Guyton AC. The surprising kidney-fluid mechanism for pressure control--its infinite gain! Hypertension. 1990;16:725–730. - PubMed
-
- Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science (New York, N.Y.) 1991;252:1813–1816. - PubMed
-
- Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension. 1992;19:I2–I8. - PubMed
-
- Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney international. Supplement. 1996;55:S35–S41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

