Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares
- PMID: 25733504
- PMCID: PMC4394816
- DOI: 10.1128/AAC.04624-14
Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares
Abstract
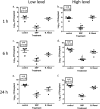
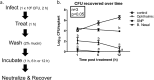
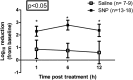
Mupirocin decolonization of nasal Staphylococcus aureus prior to surgery decreases surgical-site infections; however, treatment requires 5 days, compliance is low, and resistance occurs. In 2010, 3M Company introduced povidone-iodine (PVP-I)-based skin and nasal antiseptic (Skin and Nasal Prep [SNP]). SNP has rapid, broad-spectrum antimicrobial activity. We tested SNP's efficacy using full-thickness tissue (porcine mucosal [PM] and human skin) explant models and human subjects. Prior to or following infection with methicillin-resistant Staphylococcus aureus (MRSA) (mupirocin sensitive and resistant), explants were treated with Betadine ophthalmic preparation (Bet), SNP, or mupirocin (Bactroban nasal ointment [BN]) or left untreated. One hour posttreatment, explants were washed with phosphate-buffered saline (PBS) plus 2% mucin. One, 6, or 12 h later, bacteria were recovered and enumerated. Alternatively, following baseline sampling, human subjects applied two consecutive applications of SNP or saline to their anterior nares. One, 6, and 12 h after application of the preparation (postprep), nasal swabs were obtained, and S. aureus was enumerated. We observed that treatment of infected PM or human skin explants with SNP resulted in >2.0 log10 CFU reduction in MRSA, regardless of mupirocin sensitivity, which was significantly different from the values for BN- and Bet-treated explants and untreated controls 1 h, 6 h, and 12 h after being washed with PBS plus mucin. Swabbing the anterior nares of human subjects with SNP significantly reduced resident S. aureus compared to saline 1, 6, and 12 h postprep. Finally, pretreatment of PM explants with SNP, followed by a mucin rinse prior to infection, completely prevented MRSA infection. We conclude that SNP may be an attractive alternative for reducing the bioburden of anterior nares prior to surgery.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 29:996–1011. doi:10.1086/591861. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

