Identification and characterization of mutations conferring resistance to D-amino acids in Bacillus subtilis
- PMID: 25733611
- PMCID: PMC4403649
- DOI: 10.1128/JB.00009-15
Identification and characterization of mutations conferring resistance to D-amino acids in Bacillus subtilis
Abstract
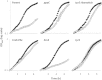
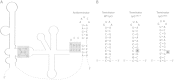
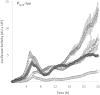
Bacteria produce d-amino acids for incorporation into the peptidoglycan and certain nonribosomally produced peptides. However, D-amino acids are toxic if mischarged on tRNAs or misincorporated into protein. Common strains of the Gram-positive bacterium Bacillus subtilis are particularly sensitive to the growth-inhibitory effects of D-tyrosine due to the absence of D-aminoacyl-tRNA deacylase, an enzyme that prevents misincorporation of D-tyrosine and other D-amino acids into nascent proteins. We isolated spontaneous mutants of B. subtilis that survive in the presence of a mixture of D-leucine, D-methionine, D-tryptophan, and D-tyrosine. Whole-genome sequencing revealed that these strains harbored mutations affecting tRNA(Tyr) charging. Three of the most potent mutations enhanced the expression of the gene (tyrS) for tyrosyl-tRNA synthetase. In particular, resistance was conferred by mutations that destabilized the terminator hairpin of the tyrS riboswitch, as well as by a mutation that transformed a tRNA(Phe) into a tyrS riboswitch ligand. The most potent mutation, a substitution near the tyrosine recognition site of tyrosyl-tRNA synthetase, improved enzyme stereoselectivity. We conclude that these mutations promote the proper charging of tRNA(Tyr), thus facilitating the exclusion of D-tyrosine from protein biosynthesis in cells that lack D-aminoacyl-tRNA deacylase.
Importance: Proteins are composed of L-amino acids. Mischarging of tRNAs with D-amino acids or the misincorporation of D-amino acids into proteins causes toxicity. This work reports on mutations that confer resistance to D-amino acids and their mechanisms of action.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. 1995. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J Biol Chem 270:15598–15606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

