Heterotrimeric NADH-oxidizing methylenetetrahydrofolate reductase from the acetogenic bacterium Acetobacterium woodii
- PMID: 25733614
- PMCID: PMC4403655
- DOI: 10.1128/JB.00048-15
Heterotrimeric NADH-oxidizing methylenetetrahydrofolate reductase from the acetogenic bacterium Acetobacterium woodii
Abstract
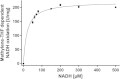
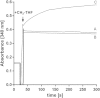
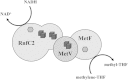
The methylenetetrahydrofolate reductase (MTHFR) of acetogenic bacteria catalyzes the reduction of methylene-THF, which is highly exergonic with NADH as the reductant. Therefore, the enzyme was suggested to be involved in energy conservation by reducing ferredoxin via electron bifurcation, followed by Na(+) translocation by the Rnf complex. The enzyme was purified from Acetobacterium woodii and shown to have an unprecedented subunit composition containing the three subunits RnfC2, MetF, and MetV. The stable complex contained 2 flavin mononucleotides (FMN), 23.5 ± 1.2 Fe and 24.5 ± 1.5 S, which fits well to the predicted six [4Fe4S] clusters in MetV and RnfC2. The enzyme catalyzed NADH:methylviologen and NADH:ferricyanide oxidoreductase activity but also methylene-tetrahydrofolate (THF) reduction with NADH as the reductant. The NADH:methylene-THF reductase activity was high (248 U/mg) and not stimulated by ferredoxin. Furthermore, reduction of ferredoxin, alone or in the presence of methylene-THF and NADH, was never observed. MetF or MetVF was not able to catalyze the methylene-THF-dependent oxidation of NADH, but MetVF could reduce methylene-THF using methyl viologen as the electron donor. The purified MTHFR complex did not catalyze the reverse reaction, the endergonic oxidation of methyl-THF with NAD(+) as the acceptor, and this reaction could not be driven by reduced ferredoxin. However, addition of protein fractions made the oxidation of methyl-THF to methylene-THF coupled to NAD(+) reduction possible. Our data demonstrate that the MTHFR of A. woodii catalyzes methylene-THF reduction according to the following reaction: NADH + methylene-THF → methyl-THF + NAD(+). The differences in the subunit compositions of MTHFRs of bacteria are discussed in the light of their different functions.
Importance: Energy conservation in the acetogenic bacterium Acetobacterium woodii involves ferredoxin reduction followed by a chemiosmotic mechanism involving Na(+)-translocating ferredoxin oxidation and a Na(+)-dependent F1Fo ATP synthase. All redox enzymes of the pathway have been characterized except the methylenetetrahydrofolate reductase (MTHFR). Here we report the purification of the MTHFR of A. woodii, which has an unprecedented heterotrimeric structure. The enzyme reduces methylene-THF with NADH. Ferredoxin did not stimulate the reaction; neither was it oxidized or reduced with NADH. Since the last enzyme with a potential role in energy metabolism of A. woodii has now been characterized, we can propose a quantitative bioenergetic scheme for acetogenesis from H2 plus CO2 in the model acetogen A. woodii.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Poehlein A, Schmidt S, Kaster A-K, Goenrich M, Vollmers J, Thürmer A, Bertsch J, Schuchmann K, Voigt B, Hecker M, Daniel R, Thauer RK, Gottschalk G, Müller V. 2012. An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7:e33439. doi:10.1371/journal.pone.0033439. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

