Glycan motif profiling reveals plasma sialyl-lewis x elevations in pancreatic cancers that are negative for sialyl-lewis A
- PMID: 25733690
- PMCID: PMC4424402
- DOI: 10.1074/mcp.M114.047837
Glycan motif profiling reveals plasma sialyl-lewis x elevations in pancreatic cancers that are negative for sialyl-lewis A
Abstract
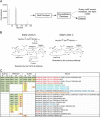
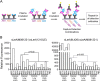
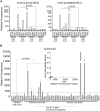
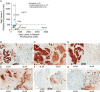
The sialyl-Lewis A (sLeA) glycan forms the basis of the CA19-9 assay and is the current best biomarker for pancreatic cancer, but because it is not elevated in ∼25% of pancreatic cancers, it is not useful for early diagnosis. We hypothesized that sLeA-low tumors secrete glycans that are related to sLeA but not detectable by CA19-9 antibodies. We used a method called motif profiling to predict that a structural isomer of sLeA called sialyl-Lewis X (sLeX) is elevated in the plasma of some sLeA-low cancers. We corroborated this prediction in a set of 48 plasma samples and in a blinded set of 200 samples. An antibody sandwich assay formed by the capture and detection of sLeX was elevated in 13 of 69 cancers that were not elevated in sLeA, and a novel hybrid assay of sLeA capture and sLeX detected 24 of 69 sLeA-low cancers. A two-marker panel based on combined sLeA and sLeX detection differentiated 109 pancreatic cancers from 91 benign pancreatic diseases with 79% accuracy (74% sensitivity and 78% specificity), significantly better than sLeA alone, which yielded 68% accuracy (65% sensitivity and 71% specificity). Furthermore, sLeX staining was evident in tumors that do not elevate plasma sLeA, including those with poorly differentiated ductal adenocarcinoma. Thus, glycan-based biomarkers could characterize distinct subgroups of patients. In addition, the combined use of sLeA and sLeX, or related glycans, could lead to a biomarker panel that is useful in the clinical diagnosis of pancreatic cancer. Précis: This paper shows that a structural isomer of the current best biomarker for pancreatic cancer, CA19-9, is elevated in the plasma of patients who are low in CA19-9, potentially enabling more comprehensive detection and classification of pancreatic cancers.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Abraham S. C., Wilentz R. E., Yeo C. J., Sohn T. A., Cameron J. L., Boitnott J. K., Hruban R. H. (2003) Pancreaticoduodenectomy (whipple resections) in patients without malignancy: Are they all “chronic pancreatitis”? Am. J. Surg. Pathol. 27, 110–120 - PubMed
-
- Adsay N. V., Bandyopadhyay S., Basturk O., Othman M., Cheng J. D., Klöppel G., Klimstra D. S. (2004) Chronic pancreatitis or pancreatic ductal adenocarcinoma? Semin. Diagn. Pathol .21, 268–276 - PubMed
-
- Kennedy T., Preczewski L., Stocker S. J., Rao S. M., Parsons W. G., Wayne J. D., Bell R. H., Talamonti M. S. (2006) Incidence of benign inflammatory disease in patients undergoing whipple procedure for clinically suspected carcinoma: A single-institution experience. Am. J. Surg. 191, 437–441 - PubMed
-
- Klöppel G., Adsay N. V. (2009) Chronic pancreatitis and the differential diagnosis versus pancreatic cancer. Arch. Pathol. Lab. Med. 133, 382–387 - PubMed
-
- Pitman M. B., Centeno B. A., Ali S. Z., Genevay M., Stelow E., Mino-Kenudson M., Castillo C. F., Schmidt C. M., Brugge W. R., Layfield L. J. (2014) Standardized terminology and nomenclature for pancreatobiliary cytology: The papanicolaou society of cytopathology guidelines. Cytojournal 11, 3. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

