GSK3- and PRMT-1-dependent modifications of desmoplakin control desmoplakin-cytoskeleton dynamics
- PMID: 25733715
- PMCID: PMC4347645
- DOI: 10.1083/jcb.201406020
GSK3- and PRMT-1-dependent modifications of desmoplakin control desmoplakin-cytoskeleton dynamics
Abstract
Intermediate filament (IF) attachment to intercellular junctions is required for skin and heart integrity, but how the strength and dynamics of this attachment are modulated during normal and pathological remodeling is poorly understood. We show that glycogen synthase kinase 3 (GSK3) and protein arginine methyltransferase 1 (PRMT-1) cooperate to orchestrate a series of posttranslational modifications on the IF-anchoring protein desmoplakin (DP) that play an essential role in coordinating cytoskeletal dynamics and cellular adhesion. Front-end electron transfer dissociation mass spectrometry analyses of DP revealed six novel serine phosphorylation sites dependent on GSK3 signaling and four novel arginine methylation sites including R2834, the mutation of which has been associated with arrhythmogenic cardiomyopathy (AC). Inhibition of GSK3 or PRMT-1 or overexpression of the AC-associated mutant R2834H enhanced DP-IF associations and delayed junction assembly. R2834H blocked the GSK3 phosphorylation cascade and reduced DP-GSK3 interactions in cultured keratinocytes and in the hearts of transgenic R2834H DP mice. Interference with this regulatory machinery may contribute to skin and heart diseases.
© 2015 Albrecht et al.
Figures
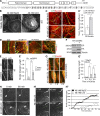

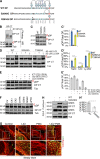
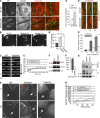
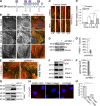

References
-
- Angst B.D., Nilles L.A., and Green K.J.. 1990. Desmoplakin II expression is not restricted to stratified epithelia. J. Cell Sci. 97:247–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR057216/AR/NIAMS NIH HHS/United States
- R37 AR043380/AR/NIAMS NIH HHS/United States
- P30AR057216/AR/NIAMS NIH HHS/United States
- GM037537/GM/NIGMS NIH HHS/United States
- R01 AR043380/AR/NIAMS NIH HHS/United States
- T32 GM008061/GM/NIGMS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- AR041836/AR/NIAMS NIH HHS/United States
- AR43380/AR/NIAMS NIH HHS/United States
- R01 CA122151/CA/NCI NIH HHS/United States
- R01 GM037537/GM/NIGMS NIH HHS/United States
- R01 AR041836/AR/NIAMS NIH HHS/United States
- R01 HL116906/HL/NHLBI NIH HHS/United States
- CA122151/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

