Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release
- PMID: 25733887
- PMCID: PMC4371942
- DOI: 10.1073/pnas.1419845112
Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release
Abstract
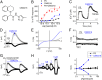
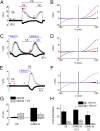
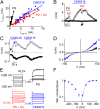
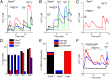
Transient receptor potential (TRP) cation channel subfamily M member 3 (TRPM3), a member of the TRP channel superfamily, was recently identified as a nociceptor channel in the somatosensory system, where it is involved in the detection of noxious heat; however, owing to the lack of potent and selective agonists, little is known about other potential physiological consequences of the opening of TRPM3. Here we identify and characterize a synthetic TRPM3 activator, CIM0216, whose potency and apparent affinity greatly exceeds that of the canonical TRPM3 agonist, pregnenolone sulfate (PS). In particular, a single application of CIM0216 causes opening of both the central calcium-conducting pore and the alternative cation permeation pathway in a membrane-delimited manner. CIM0216 evoked robust calcium influx in TRPM3-expressing somatosensory neurons, and intradermal injection of the compound induced a TRPM3-dependent nocifensive behavior. Moreover, CIM0216 elicited the release of the peptides calcitonin gene-related peptide (CGRP) from sensory nerve terminals and insulin from isolated pancreatic islets in a TRPM3-dependent manner. These experiments identify CIM0216 as a powerful tool for use in investigating the physiological roles of TRPM3, and indicate that TRPM3 activation in sensory nerve endings can contribute to neurogenic inflammation.
Keywords: TRP channel; TRPM3; nociceptor; peptide release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1(2):85–92. - PubMed
-
- Montell C, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9(2):229–231. - PubMed
-
- Vriens J, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70(3):482–494. - PubMed
-
- Wagner TF, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10(12):1421–1430. - PubMed
-
- Vriens J, et al. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat Chem Biol. 2014;10(3):188–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

