Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy
- PMID: 25733888
- PMCID: PMC4371943
- DOI: 10.1073/pnas.1502214112
Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy
Abstract
The amyloid state of polypeptides is a stable, highly organized structural form consisting of laterally associated β-sheet protofilaments that may be adopted as an alternative to the functional, native state. Identifying the balance of forces stabilizing amyloid is fundamental to understanding the wide accessibility of this state to peptides and proteins with unrelated primary sequences, various chain lengths, and widely differing native structures. Here, we use four-dimensional electron microscopy to demonstrate that the forces acting to stabilize amyloid at the atomic level are highly anisotropic, that an optimized interbackbone hydrogen-bonding network within β-sheets confers 20 times more rigidity on the structure than sequence-specific sidechain interactions between sheets, and that electrostatic attraction of protofilaments is only slightly stronger than these weak amphiphilic interactions. The potential biological relevance of the deposition of such a highly anisotropic biomaterial in vivo is discussed.
Keywords: 4D electron diffraction; nanomechanics; proteins; structural dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
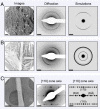
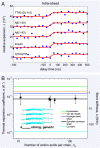
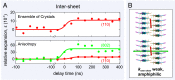
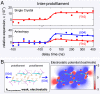
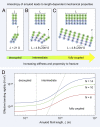
References
-
- Knowles TP, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nat Nanotechnol. 2011;6(8):469–479. - PubMed
-
- Sunde M, et al. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273(3):729–739. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Gras SL, et al. Functionalised amyloid fibrils for roles in cell adhesion. Biomaterials. 2008;29(11):1553–1562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

