Impact of Xpert MTB/RIF on Antiretroviral Therapy-Associated Tuberculosis and Mortality: A Pragmatic Randomized Controlled Trial
- PMID: 25734106
- PMCID: PMC4324195
- DOI: 10.1093/ofid/ofu038
Impact of Xpert MTB/RIF on Antiretroviral Therapy-Associated Tuberculosis and Mortality: A Pragmatic Randomized Controlled Trial
Abstract
Introduction: GeneXpert® MTB/RIF (Xpert) is now widely distributed in high human immunodeficiency virus (HIV)/tuberculosis (TB)-burden countries. Yet, whether the test improves patient-important outcomes within HIV treatment programs in limited resource settings is unknown.
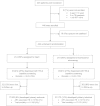
Methods: To investigate whether use of Xpert for TB screening prior to initiation of antiretroviral treatment (ART) improves patient-important outcomes, in a pragmatic randomized controlled trial we assigned 424 patients to Xpert or fluorescence sputum smear microscopy (FM) at ART initiation. The primary endpoint was a composite of 3-month mortality and ART-associated TB.
Results: There was no difference in overall TB diagnosis at ART initiation (20% [n = 43] Xpert vs 21% [n = 45] FM; P = .80), with most patients in both groups treated empirically. There was no difference in time to TB treatment initiation {5 days (interquartile range [IQR], 3-13) vs 8 days [IQR, 3-23; P = .26]} or loss to follow-up (32 [15%] vs 38 [18%]; P = 0.38). Although a nonsignificant reduction in mortality occurred in the Xpert group (11 [6%] vs 17 [10%]; 95% CI, -9% to 2%; P = .19), there was no difference in the composite outcome (9% [n = 17] Xpert vs 12% [n = 21] FM; difference -3%; 95% CI, -9% to 4%).
Conclusions: Among HIV-infected initiating ART, centralized TB screening with Xpert did not reduce the rate of ART-associated TB and mortality, compared with fluorescence microscopy.
Keywords: ART-associated TB; GeneXpert MTB/RIF; HIV; screening; tuberculosis.
Figures
References
-
- UN Joint Programme on HIV/AIDS, Treatment 2015. 2013. July. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspubl... . Accessed 13 August 2013.
-
- Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. - PubMed
-
- FIND. WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://www.stoptb.org/wg/gli/assets/documents/map/1/atlas.html . Accessed 20 August 2013.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources