Cholinesterase inhibitors for rarer dementias associated with neurological conditions
- PMID: 25734590
- PMCID: PMC10644993
- DOI: 10.1002/14651858.CD009444.pub3
Cholinesterase inhibitors for rarer dementias associated with neurological conditions
Abstract
Background: Rarer dementias include Huntington's disease (HD), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), frontotemporal dementia (FTD), dementia in multiple sclerosis (MS) and progressive supranuclear palsy (PSP). Cholinesterase inhibitors, including donepezil, galantamine and rivastigmine, are considered to be the first-line medicines for Alzheimer's disease and some other dementias, such as dementia in Parkinson's disease. Cholinesterase inhibitors are hypothesised to work by inhibiting the enzyme acetylcholinesterase (AChE) which breaks down the neurotransmitter acetylcholine. Cholinesterase inhibitors may also lead to clinical improvement for rarer dementias associated with neurological conditions.
Objectives: To assess the efficacy and safety of cholinesterase inhibitors for cognitive impairment or dementia associated with neurological conditions.
Search methods: We searched the Cochrane Dementia and Cognitive Improvement Group's Specialised Register, CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS, several trial registries and grey literature sources in August 2013.
Selection criteria: We included randomised, double-blind, controlled trials assessing the efficacy of treatment of rarer dementias associated with neurological conditions with currently marketed cholinesterase inhibitors.
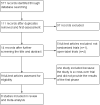
Data collection and analysis: Two review authors independently assessed eligibility and quality of trials, and extracted data. We used the standard methodological procedures of the Cochrane Collaboration.
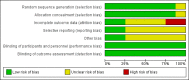
Main results: We included eight RCTs involving 567 participants. Six studies used a simple parallel-group design; the other two consisted of an open-label treatment period followed by a randomised phase. All trials were well concealed for allocation and double-blind, however the sample sizes of most trials were small. All trials used placebo as control. We performed meta-analyses for some outcomes in patients with MS. For all other conditions, results are presented narratively.Two trials included patients with HD; one found that cholinesterase inhibitor use in the short-term had no statistically significant impact on the cognitive portion of the Alzheimer Disease Assessment Scale (ADAS-Cog; 1 study, WMD 1.00, 95% CI -1.66 to 3.66, P = 0.46; low quality evidence), Unified Huntington's Disease Rating Scale (UHDRS) Verbal Fluency Test (1 study, WMD -1.20, 95% CI -7.97 to 5.57, P = 0.73; low quality evidence), UHDRS Symbol Digit Modalities Test (SDMT; 1 study, WMD 2.70, 95% CI -0.95 to 6.35, P = 0.15; low quality evidence) and other psychometric tests. The other study found that cholinesterase inhibitor use in the medium-term improved the results of the verbal fluency test (1 study, WMD 6.43, 95% CI 0.66 to 12.20, P = 0.03; moderate quality evidence) and California Verbal Learning Test - Second Edition (CVLT-II) Recognition Task (1 study, WMD 2.42, 95% CI 0.17 to 4.67, P = 0.04; moderate quality evidence). There was no statistically significant difference between groups on the SDMT (1 study, WMD -0.31, 95% CI -7.77 to 7.15, P = 0.94; moderate quality evidence), CVLT-II trials 1-5 (1 study, WMD -2.09, 95% CI -11.65 to 7.47, P = 0.67; moderate quality evidence), short-delay recall (1 study, WMD 0.35, 95% CI -2.87 to 3.57, P = 0.83; moderate quality evidence), or long-delay recall (1 study, WMD -0.14, 95% CI -3.08 to 2.80, P = 0.93; moderate quality evidence), and other psychometric tests.Four trials included patients with MS; one found no differences between the cholinesterase inhibitors (short-term) and placebo groups on the Wechsler Memory Scales general memory score (1 study, WMD 0.90, 95% CI -0.52 to 2.32, P = 0.22; low quality evidence). The three other trials found that, in the medium-term - cholinesterase inhibitors improved the clinician's impression of cognitive change (2 studies, OR 1.96, 95% CI 1.06 to 3.62, P = 0.03; high quality evidence). However, the treatment effect on other aspects of cognitive change were unclear, measured by the Selective Reminding Test (3 studies, WMD 1.47, 95% CI -0.39 to 3.32, P = 0.12; high quality evidence), patient's self-reported impression of memory change (2 studies, OR 1.67, 95% CI 0.93 to 3.00, P = 0.08; high quality evidence) and cognitive change (1 study, OR 0.95, 95% CI 0.45 to 1.98, P = 0.89; high quality evidence), clinician's impression of memory change (1 study, OR 1.50, 95% CI 0.59 to 3.84, P = 0.39; moderate quality evidence), other psychometric tests, and activities of daily living - patient reported impact of multiple sclerosis activities (1 study, WMD -1.18, 95% CI -3.02 to 0.66, P = 0.21; low quality evidence).One study on patients with CADASIL found a beneficial effect of cholinesterase inhibitors on the Executive interview, and Trail Making Test parts A and B. The impact of cholinesterase inhibitors on the Vascular ADAS-Cog score (1 study, WMD 0.04, 95% CI -1.57 to 1.65, P = 0.96; high quality evidence), the Clinical Dementia Rating Scale Sum of Boxes (1 study, WMD -0.09, 95% CI -0.48 to 0.03, P = 0.65; high quality evidence) Disability Assessment for Dementia scale (1 study, WMD 0.58, 95% CI -2.72 to 3.88, P = 0.73; moderate quality evidence), and other measures was unclearOne study included patients with FTD. This trial consisted of an open-label treatment period followed by a randomised, double-blind, placebo-controlled phase. No data of primary outcomes were reported in this study.In the included studies, the most common side effect was gastrointestinal symptoms. For all conditions, compared to the treatment group, the placebo group experienced significantly less nausea (6 studies, 44/257 vs. 22/246, OR 2.10, 95% CI 1.22 to 3.62, P = 0.007; high quality evidence), diarrhoea (6 studies, 40/257 vs. 13/246, OR 3.26, 95% CI 1.72 to 6.19, P = 0.0003; moderate quality evidence) and vomiting (3 studies, 17/192 vs. 3/182, OR 5.76, 95% CI 1.67 to 19.87, P = 0.006; moderate quality evidence).
Authors' conclusions: The sample sizes of most included trials were small, and some of the results were extracted from only one study. There were no poolable data for HD, CADASIL and FTD patients and there were no results for patients with PSP. Current evidence shows that the efficacy on cognitive function and activities of daily living of cholinesterase inhibitors in people with HD, CADASIL, MS, PSP or FTD is unclear, although cholinesterase inhibitors are associated with more gastrointestinal side effects compared with placebo.
Conflict of interest statement
Ying Li ‐ None known Shan Hai ‐ None known Yan Zhou ‐ None known Bi Rong Dong ‐ None known
Figures








Update of
References
References to studies included in this review
Cubo 2006 {published data only}
-
- Cubo E, Shannon KM, Tracy D, Jaglin JA, Bernard BA, Wuu J, et al. Effect of donepezil on motor and cognitive function in Huntington disease. Neurology 2006;67(7):1268‐71. - PubMed
Dichgans 2008 {published data only}
-
- Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double‐blind trial in CADASIL. Lancet Neurology 2008; Vol. 7, issue 4:310‐8. - PubMed
Kertesz 2008 {published data only}
-
- Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dementia and Geriatric Cognitive Disorders 2008; Vol. 25, issue 2:178‐85. - PubMed
Krupp 2004 {published data only}
-
- Krupp LB, Christodoulou C, Melville P, Scherl WF, MacAllister WS, Elkins LE. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology 2004; Vol. 63, issue 9:1579‐85. - PubMed
Krupp 2011 {published data only}
Mäurer 2013 {published data only}
-
- Maurer M, Ortler S, Baier M, Meergans M, Scherer P, Hofmann W, et al. Randomised multicentre trial on safety and efficacy of rivastigmine in cognitively impaired multiple sclerosis patients. Multiple Sclerosis 2013; Vol. 19, issue 5:631‐8. - PubMed
Sešok 2014 {published data only}
-
- Sešok S, Bolle N, Kobal J, Bucik V, Vodušek DB. Cognitive function in early clinical phase huntington disease after rivastigmine treatment. Psychiatria Danubina 2014;26(3):239‐48. - PubMed
Shaygannejad 2008 {published data only}
-
- Shaygannejad V, Janghorbani M, Ashtari F, Zanjani HA, Zakizade N. Effects of rivastigmine on memory and cognition in multiple sclerosis. Canadian Journal of Neurological Sciences 2008; Vol. 35, issue 4:476‐81. - PubMed
References to studies excluded from this review
de Tommaso 2004 {published data only}
-
- Tommaso M, Specchio N, Sciruicchio V, Difruscolo O, Specchio LM. Effects of rivastigmine on motor and cognitive impairment in Huntington's disease. Movement Disorders 2004;19(12):1516‐18. - PubMed
de Tommaso 2007 {published data only}
-
- Tommaso M, Difruscolo O, Sciruicchio V, Specchio N, Livrea P. Two years' follow‐up of rivastigmine treatment in Huntington disease. Clinical Neuropharmacology 2007;30(1):43‐6. - PubMed
Fabbrini 2001 {published data only}
-
- Fabbrini G, Barbanti P, Bonifati V, Colosimo C, Gasparini M, Vanacore N, Meco G. Donepezil in the treatment of progressive supranuclear palsy. Acta Neurologica Scandinavica 2001;103(2):123‐5. - PubMed
Greene 2000 {published data only}
-
- Greene YM, Tariot PN, Wishart H, Cox C, Holt CJ, Schwid S, et al. A 12‐week, open trial of donepezil hydrochloride in patients with multiple sclerosis and associated cognitive impairments. Journal of clinical psychopharmacology 2000;20(3):350‐6. - PubMed
Litvan 2001 {published data only}
-
- Litvan I, Phipps M, Pharr VL, Hallett M, Grafman J, Salazar A. Randomized placebo‐controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 2001; Vol. 57, issue 3:467‐73. - PubMed
Moretti 2004 {published data only}
-
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open‐label study. Drugs & aging 2004;21(14):931‐7. - PubMed
Additional references
Baskys 2012
-
- Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Experimental Gerontology 2012;47(11):887‐91. - PubMed
Boeve 2012
-
- Boeve BF. Progressive supranuclear palsy. Parkinsonism Related Disorders 2012;18(Suppl 1):S192‐4. - PubMed
Brun 1994
Cacabelos 2008
-
- Cacabelos R. Pharmacogenomics and therapeutic prospects in dementia. European Archives of Psychiatry and Clinical Neuroscience 2008;258(Suppl 1):28‐47. - PubMed
Chiaravalloti 2008
-
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurology 2008;7(12):1139‐51. - PubMed
Choi 2010
Cochrane Handbook
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Fukutake 2011
-
- Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): from discovery to gene identification. Journal of Stroke and Cerebrovascular Diseases 2011;20(2):85‐93. - PubMed
Hansen 2008
Higgins 2003
Hoppitt 2011
Joutel 1996
-
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 1996;383(6602):707‐10. - PubMed
Keverne 2007
-
- Keverne JS, Low WC, Ziabreva I, Court JA, Oakley AE, Kalaria RN. Cholinergic neuronal deficits in CADASIL. Stroke 2007;38(1):188‐91. - PubMed
Kurz 2009
Lanctôt 2009
Levine 2011
Linder 2010
-
- Linder J, Stenlund H, Forsgren L. Incidence of Parkinson's disease and parkinsonism in northern Sweden: a population‐based study. Movement Disorders 2010;25(3):341‐8. - PubMed
Litvan 1996
-
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47(1):1‐9. - PubMed
Martin 2013
-
- Martin P, Maëlenn G, Matthew P, Alzheimer’s Disease International. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050. http://www.alz.co.uk/research/GlobalImpactDementia2013.pdf December 2013.
Mesulam 2003
-
- Mesulam M, Siddique T, Cohen B. Cholinergic denervation in a pure multi‐infarct state: observations on CADASIL. Neurology 2003;60(7):1183‐5. - PubMed
Mollenhauer 2010
Narayan 2012
Neary 1998
-
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51(6):1546‐54. - PubMed
NICE 2011
-
- National Institute for Health and Clinical Excellence (NICE). Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease.. London, UK: National Institute for Health and Clinical Excellence (NICE), 2011.
Nunnemann 2012
-
- Nunnemann S, Kurz A, Leucht S, Diehl‐Schmid J. Caregivers of patients with frontotemporal lobar degeneration: a review of burden, problems, needs, and interventions. International Psychogeriatrics/IPA 2012;24(9):1368‐86. - PubMed
Odawara 2003
Paulsen 2011
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. - PubMed
Premi 2012
-
- Premi E, Padovani A, Borroni B. Frontotemporal Lobar Degeneration. Advances in Experimental Medicine and Biology 2012;724:114‐27. - PubMed
Pringsheim 2012
-
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta‐analysis. Movement Disorders 2012;27(9):1083‐91. - PubMed
Rabinovici 2010
Rahn 2012
Rascovsky 2011
Razvi 2005
Rolinski 2012
Ruberg 1987
-
- Ruberg M, Villageois A, Bonnet AM, Pillon B, Rieger F, Agid Y. Acetylcholinesterase and butyrylcholinesterase activity in the cerebrospinal fluid of patients with neurodegenerative diseases involving cholinergic systems. Journal of Neurology, Neurosurgery and Psychiatry 1987;50(5):538‐43. - PMC - PubMed
Tayeb 2012
-
- Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer's disease: beyond cholinesterase inhibitors. Pharmacology & Therapeutics 2012;134(1):8‐25. - PubMed
WHO 2010
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision Version (ICD‐10). Geneva,Switzerland: WHO Press, 2010.
References to other published versions of this review
Chabriat 1995
-
- Chabriat H, Vahedi K, Iba‐Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet 1995;346(8980):934‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

