Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data
- PMID: 25734701
- PMCID: PMC4348415
- DOI: 10.1371/journal.pbio.1002082
Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data
Abstract
The immunity of a host population against specific influenza A strains can influence a number of important biological processes, from the emergence of new virus strains to the effectiveness of vaccination programmes. However, the development of an individual's long-lived antibody response to influenza A over the course of a lifetime remains poorly understood. Accurately describing this immunological process requires a fundamental understanding of how the mechanisms of boosting and cross-reactivity respond to repeated infections. Establishing the contribution of such mechanisms to antibody titres remains challenging because the aggregate effect of immune responses over a lifetime are rarely observed directly. To uncover the aggregate effect of multiple influenza infections, we developed a mechanistic model capturing both past infections and subsequent antibody responses. We estimated parameters of the model using cross-sectional antibody titres to nine different strains spanning 40 years of circulation of influenza A(H3N2) in southern China. We found that "antigenic seniority" and quickly decaying cross-reactivity were important components of the immune response, suggesting that the order in which individuals were infected with influenza strains shaped observed neutralisation titres to a particular virus. We also obtained estimates of the frequency and age distribution of influenza infection, which indicate that although infections became less frequent as individuals progressed through childhood and young adulthood, they occurred at similar rates for individuals above age 30 y. By establishing what are likely to be important mechanisms driving epochal trends in population immunity, we also identified key directions for future studies. In particular, our results highlight the need for longitudinal samples that are tested against multiple historical strains. This could lead to a better understanding of how, over the course of a lifetime, fast, transient antibody dynamics combine with the longer-term immune responses considered here.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
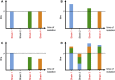
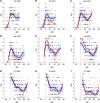

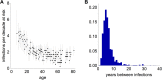

References
-
- Ferguson NM, Galvani AP, Bush RM (2003) Ecological and immunological determinants of influenza evolution. Nature 422: 428–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

