A novel bipartite centrosome coordinates the apicomplexan cell cycle
- PMID: 25734885
- PMCID: PMC4348508
- DOI: 10.1371/journal.pbio.1002093
A novel bipartite centrosome coordinates the apicomplexan cell cycle
Abstract
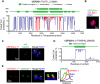
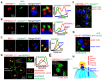
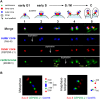
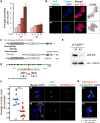
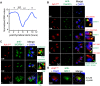
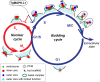
Apicomplexan parasites can change fundamental features of cell division during their life cycles, suspending cytokinesis when needed and changing proliferative scale in different hosts and tissues. The structural and molecular basis for this remarkable cell cycle flexibility is not fully understood, although the centrosome serves a key role in determining when and how much replication will occur. Here we describe the discovery of multiple replicating core complexes with distinct protein composition and function in the centrosome of Toxoplasma gondii. An outer core complex distal from the nucleus contains the TgCentrin1/TgSfi1 protein pair, along with the cartwheel protein TgSas-6 and a novel Aurora-related kinase, while an inner core closely aligned with the unique spindle pole (centrocone) holds distant orthologs of the CEP250/C-Nap protein family. This outer/inner spatial relationship of centrosome cores is maintained throughout the cell cycle. When in metaphase, the duplicated cores align to opposite sides of the kinetochores in a linear array. As parasites transition into S phase, the cores sequentially duplicate, outer core first and inner core second, ensuring that each daughter parasite inherits one copy of each type of centrosome core. A key serine/threonine kinase distantly related to the MAPK family is localized to the centrosome, where it restricts core duplication to once per cycle and ensures the proper formation of new daughter parasites. Genetic analysis of the outer core in a temperature-sensitive mutant demonstrated this core functions primarily in cytokinesis. An inhibition of ts-TgSfi1 function at high temperature caused the loss of outer cores and a severe block to budding, while at the same time the inner core amplified along with the unique spindle pole, indicating the inner core and spindle pole are independent and co-regulated. The discovery of a novel bipartite organization in the parasite centrosome that segregates the functions of karyokinesis and cytokinesis provides an explanation for how cell cycle flexibility is achieved in apicomplexan life cycles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Parasite biology: Dual-core centrosomes power cell division.Nat Rev Microbiol. 2015 May;13(5):252. doi: 10.1038/nrmicro3470. Epub 2015 Mar 23. Nat Rev Microbiol. 2015. PMID: 25798750 No abstract available.
-
Apicomplexan cell cycle flexibility: centrosome controls the clutch.Trends Parasitol. 2015 Jun;31(6):229-30. doi: 10.1016/j.pt.2015.04.003. Epub 2015 Apr 19. Trends Parasitol. 2015. PMID: 25899747 Free PMC article.
References
-
- Sogin ML, Silberman JD (1998) Evolution of the protists and protistan parasites from the perspective of molecular systematics. Int J Parasitol 28: 11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

