Direct catalytic enantio- and diastereoselective ketone aldol reactions of isocyanoacetates
- PMID: 25735645
- PMCID: PMC4678506
- DOI: 10.1002/anie.201411852
Direct catalytic enantio- and diastereoselective ketone aldol reactions of isocyanoacetates
Abstract
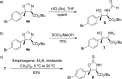
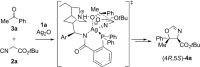
A catalytic asymmetric aldol addition/cyclization reaction of unactivated ketones with isocyanoacetate pronucleophiles has been developed. A quinine-derived aminophosphine precatalyst and silver oxide were found to be an effective binary catalyst system and promoted the reaction to afford chiral oxazolines possessing a fully substituted stereocenter with good diastereoselectivities and excellent enantioselectivities.
Keywords: aldol reaction; asymmetric catalysis; enantioselectivity; isocyanoacetates; oxazolines.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- For reviews on asymmetric aldol reactions, see:
-
- Mahrwald R. Modern Aldol Reactions. Weinheim: Wiley-VCH; 2004.
-
- Palomo C, Oiarbide M, García JM. Chem. Soc. Rev. 2004;33:65–75. - PubMed
-
- Geary LM, Hultin PG. Tetrahedron: Asymmetry. 2009;20:131–173.
LinkOut - more resources
Full Text Sources
Other Literature Sources

