Exposure to the complement C5b-9 complex sensitizes 661W photoreceptor cells to both apoptosis and necroptosis
- PMID: 25735751
- PMCID: PMC4348505
- DOI: 10.1007/s10495-015-1091-7
Exposure to the complement C5b-9 complex sensitizes 661W photoreceptor cells to both apoptosis and necroptosis
Abstract
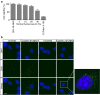
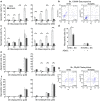
The loss of photoreceptors is the defining characteristic of many retinal degenerative diseases, but the mechanisms that regulate photoreceptor cell death are not fully understood. Here we have used the 661W cone photoreceptor cell line to ask whether exposure to the terminal complement complex C5b-9 induces cell death and/or modulates the sensitivity of these cells to other cellular stressors. 661W cone photoreceptors were exposed to complete normal human serum following antibody blockade of CD59. Apoptosis induction was assessed morphologically, by flow cytometry, and on western blotting by probing for cleaved PARP and activated caspase-3. Necroptosis was assessed by flow cytometry and Sirtuin 2 inhibition using 2-cyano-3-[5-(2,5-dichlorophenyl)-2-furyl]-N-5-quinolinylacrylamide (AGK2). The sensitivity of 661W cells to ionomycin, staurosporine, peroxide and chelerythrine was also investigated, with or without prior formation of C5b-9. 661W cells underwent apoptotic cell death following exposure to C5b-9, as judged by poly(ADP-ribose) polymerase 1 cleavage and activation of caspase-3. We also observed apoptotic cell death in response to staurosporine, but 661W cells were resistant to both ionomycin and peroxide. Interestingly, C5b-9 significantly increased 661W sensitivity to staurosporine-induced apoptosis and necroptosis. These studies show that low levels of C5b-9 on 661W cells can induce apoptosis, and that C5b-9 specifically sensitizes 661W cells to certain apoptotic and necroptotic pathways. Our observations provide new insight into the potential role of the complement system in photoreceptor loss, with implications for the molecular aetiology of retinal disease.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

