A comprehensive review of glycosylated bacterial natural products
- PMID: 25735878
- PMCID: PMC4560691
- DOI: 10.1039/c4cs00426d
A comprehensive review of glycosylated bacterial natural products
Abstract
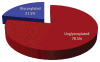
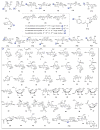
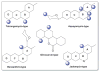
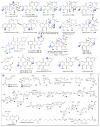
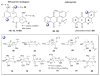
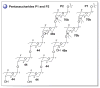
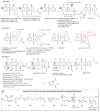
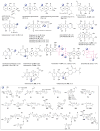
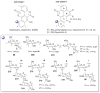
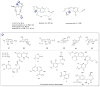
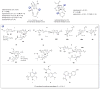
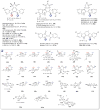
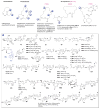
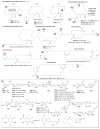
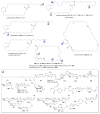
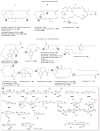
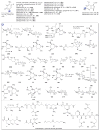
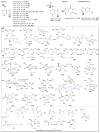
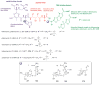
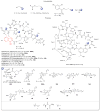
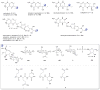
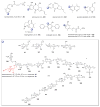
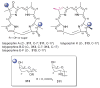
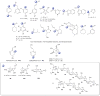
A systematic analysis of all naturally-occurring glycosylated bacterial secondary metabolites reported in the scientific literature up through early 2013 is presented. This comprehensive analysis of 15 940 bacterial natural products revealed 3426 glycosides containing 344 distinct appended carbohydrates and highlights a range of unique opportunities for future biosynthetic study and glycodiversification efforts.
Figures
































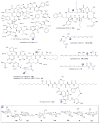
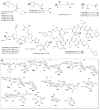
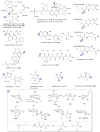


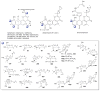









References
-
- Kapoor VK, Kaur A. Mini-Rev Med Chem. 2013;13:584–596. - PubMed
-
- Desmet T, Soetaert W, Bojarova P, Kren V, Dijkhuizen L, Eastwick-Field V, Schiller A. Chem – Eur J. 2012;18:10786–10801. - PubMed
-
- La Ferla B, Airoldi C, Zona C, Orsato A, Cardona F, Merlo S, Sironi E, D’Orazio G, Nicotra F. Nat Prod Rep. 2011;28:630–648. - PubMed
-
- Thuan NH, Sohng JK. J Ind Microbiol Biotechnol. 2013;40:1329–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

