Effects of pre-analytical processes on blood samples used in metabolomics studies
- PMID: 25736245
- PMCID: PMC4471316
- DOI: 10.1007/s00216-015-8565-x
Effects of pre-analytical processes on blood samples used in metabolomics studies
Abstract
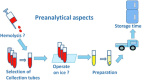
Every day, analytical and bio-analytical chemists make sustained efforts to improve the sensitivity, specificity, robustness, and reproducibility of their methods. Especially in targeted and non-targeted profiling approaches, including metabolomics analysis, these objectives are not easy to achieve; however, robust and reproducible measurements and low coefficients of variation (CV) are crucial for successful metabolomics approaches. Nevertheless, all efforts from the analysts are in vain if the sample quality is poor, i.e. if preanalytical errors are made by the partner during sample collection. Preanalytical risks and errors are more common than expected, even when standard operating procedures (SOP) are used. This risk is particularly high in clinical studies, and poor sample quality may heavily bias the CV of the final analytical results, leading to disappointing outcomes of the study and consequently, although unjustified, to critical questions about the analytical performance of the approach from the partner who provided the samples. This review focuses on the preanalytical phase of liquid chromatography-mass spectrometry-driven metabolomics analysis of body fluids. Several important preanalytical factors that may seriously affect the profile of the investigated metabolome in body fluids, including factors before sample collection, blood drawing, subsequent handling of the whole blood (transportation), processing of plasma and serum, and inadequate conditions for sample storage, will be discussed. In addition, a detailed description of latent effects on the stability of the blood metabolome and a suggestion for a practical procedure to circumvent risks in the preanalytical phase will be given.
Figures


References
-
- Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. - PubMed
-
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu JD, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao XH, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. - PMC - PubMed
-
- Lokhov PG, Dashtiev MI, Moshkovskii SA, Archakov AI. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics. 2010;6(1):156–163.
-
- Huang Q, Tan YX, Yin PY, Ye GZ, Gao P, Lu X, Wang HY, Xu GW. Metabolic Characterization of Hepatocellular Carcinoma Using Nontargeted Tissue Metabolomics. Cancer Res. 2013;73(16):4992–5002. - PubMed
-
- Duarte IF, Rocha CM, Gil AM. Metabolic profiling of biofluids: potential in lung cancer screening and diagnosis. Expert Rev Mol Diagn. 2013;13(7):737–748. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

