Prevention of carcinogen and inflammation-induced dermal cancer by oral rapamycin includes reducing genetic damage
- PMID: 25736275
- PMCID: PMC4417432
- DOI: 10.1158/1940-6207.CAPR-14-0313-T
Prevention of carcinogen and inflammation-induced dermal cancer by oral rapamycin includes reducing genetic damage
Abstract
Cancer prevention is a cost-effective alternative to treatment. In mice, the mTOR inhibitor rapamycin prevents distinct spontaneous, noninflammatory cancers, making it a candidate broad-spectrum cancer prevention agent. We now show that oral microencapsulated rapamycin (eRapa) prevents skin cancer in dimethylbenz(a)anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) carcinogen-induced, inflammation-driven carcinogenesis. eRapa given before DMBA/TPA exposure significantly increased tumor latency, reduced papilloma prevalence and numbers, and completely inhibited malignant degeneration into squamous cell carcinoma. Rapamycin is primarily an mTORC1-specific inhibitor, but eRapa did not reduce mTORC1 signaling in skin or papillomas, and did not reduce important proinflammatory factors in this model, including p-Stat3, IL17A, IL23, IL12, IL1β, IL6, or TNFα. In support of lack of mTORC1 inhibition, eRapa did not reduce numbers or proliferation of CD45(-)CD34(+)CD49f(mid) skin cancer initiating stem cells in vivo and marginally reduced epidermal hyperplasia. Interestingly, eRapa reduced DMBA/TPA-induced skin DNA damage and the hras codon 61 mutation that specifically drives carcinogenesis in this model, suggesting reduction of DNA damage as a cancer prevention mechanism. In support, cancer prevention and DNA damage reduction effects were lost when eRapa was given after DMBA-induced DNA damage in vivo. eRapa afforded picomolar concentrations of rapamycin in skin of DMBA/TPA-exposed mice, concentrations that also reduced DMBA-induced DNA damage in mouse and human fibroblasts in vitro. Thus, we have identified DNA damage reduction as a novel mechanism by which rapamycin can prevent cancer, which could lay the foundation for its use as a cancer prevention agent in selected human populations.
©2015 American Association for Cancer Research.
Conflict of interest statement
The authors have no relevant financial conflicts of interest to declare.
Figures



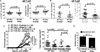

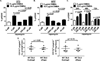
Similar articles
-
Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate.Cancer Prev Res (Phila). 2011 Jul;4(7):1011-20. doi: 10.1158/1940-6207.CAPR-10-0375. Cancer Prev Res (Phila). 2011. PMID: 21733825 Free PMC article.
-
Genetic deletion of TNFα inhibits ultraviolet radiation-induced development of cutaneous squamous cell carcinomas in PKCε transgenic mice via inhibition of cell survival signals.Carcinogenesis. 2016 Jan;37(1):72-80. doi: 10.1093/carcin/bgv162. Epub 2015 Nov 19. Carcinogenesis. 2016. PMID: 26586792 Free PMC article.
-
A course of very small doses of DMBA, each of them allegedly with no promoting potency, acts with clear synergistic effect as a strong promoter of DMBA-initiated mouse skin carcinogenesis. A comparison of the tumorigenic and carcinogenic effects of DMBA (7,12-dimethylbenz-alpha-anthracene) and TPA (12-O-tetradecanoyl-phorbol-13-acetate) used as initiators and promoters in classical two-stage experimental protocols.APMIS Suppl. 1994;41:1-38. APMIS Suppl. 1994. PMID: 7946481
-
Modification of multistage skin carcinogenesis in mice.Prog Exp Tumor Res. 1991;33:192-229. doi: 10.1159/000419252. Prog Exp Tumor Res. 1991. PMID: 1902960 Review.
-
Chemically induced carcinogenesis in rodent models of aging: assessing organismal resilience to genotoxic stressors in geroscience research.Geroscience. 2019 Apr;41(2):209-227. doi: 10.1007/s11357-019-00064-4. Epub 2019 Apr 29. Geroscience. 2019. PMID: 31037472 Free PMC article. Review.
Cited by
-
Biphasic Rapamycin Effects in Lymphoma and Carcinoma Treatment.Cancer Res. 2017 Jan 15;77(2):520-531. doi: 10.1158/0008-5472.CAN-16-1140. Epub 2016 Oct 13. Cancer Res. 2017. PMID: 27737881 Free PMC article.
-
Rapamycin Extends Life Span in ApcMin/+ Colon Cancer FAP Model.Clin Colorectal Cancer. 2021 Mar;20(1):e61-e70. doi: 10.1016/j.clcc.2020.08.006. Epub 2020 Sep 15. Clin Colorectal Cancer. 2021. PMID: 33132009 Free PMC article.
-
Immune-Stimulatory Effects of Rapamycin Are Mediated by Stimulation of Antitumor γδ T Cells.Cancer Res. 2016 Oct 15;76(20):5970-5982. doi: 10.1158/0008-5472.CAN-16-0091. Epub 2016 Aug 28. Cancer Res. 2016. PMID: 27569211 Free PMC article.
-
Reduction in squamous cell carcinomas in mouse skin by dietary zinc supplementation.Cancer Med. 2016 Aug;5(8):2032-42. doi: 10.1002/cam4.768. Epub 2016 May 17. Cancer Med. 2016. PMID: 27185213 Free PMC article.
-
Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma.Cancer Res. 2016 Dec 1;76(23):6964-6974. doi: 10.1158/0008-5472.CAN-16-0258. Epub 2016 Sep 26. Cancer Res. 2016. PMID: 27671674 Free PMC article.
References
-
- Society AC. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012.
Publication types
MeSH terms
Substances
Grants and funding
- F30 CA180377/CA/NCI NIH HHS/United States
- CA180377/CA/NCI NIH HHS/United States
- CA54174/CA/NCI NIH HHS/United States
- R01 CA193835/CA/NCI NIH HHS/United States
- CA170491/CA/NCI NIH HHS/United States
- TL1TR001119/TR/NCATS NIH HHS/United States
- T35 AG038048/AG/NIA NIH HHS/United States
- R01 CA164122/CA/NCI NIH HHS/United States
- R21 CA170491/CA/NCI NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- UL1 TR001120/TR/NCATS NIH HHS/United States
- AG038048/AG/NIA NIH HHS/United States
- TL1 TR001119/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

