Effects of neurotrophin receptor-mediated MAGE homology on proliferation and odontoblastic differentiation of mouse dental pulp cells
- PMID: 25736627
- PMCID: PMC6496191
- DOI: 10.1111/cpr.12171
Effects of neurotrophin receptor-mediated MAGE homology on proliferation and odontoblastic differentiation of mouse dental pulp cells
Abstract
Objectives: This study aimed to investigate effects of neurotrophin receptor-mediated melanoma antigen-encoding gene homology (NRAGE) on proliferation and odontoblastic differentiation of mouse dental pulp cells (mDPCs).
Materials and methods: Mouse dental pulp cells were infected with recombinant lentivirus to stably knockdown expression of NRAGE, and biological effects of NRAGE on the cells were detected. Proliferation and odontoblastic differentiation of mDPCs were observed. Simultaneously, mRNA and protein levels of NRAGE and nuclear factor-κB (NF-κB) protein expression were detected. Immunofluorescence assay was used to detect expression and location of NRAGE and NF-κB.
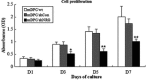
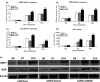
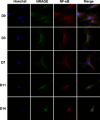
Results: NRAGE mRNA and protein levels reduced significantly after mDPC odontoblastic induction. Knockdown of NRAGE inhibited the proliferation of mDPCs. However, knockdown of NRAGE enhanced their odontoblastic differentiation with up-regulated ALPase activity. It also promoted mineral nodule formation as well as mRNA and protein expressions of ALP, DSPP and DMP1. Protein levels of NF-κB/p50 significantly increased, whereas NF-κB/p105 protein expression decreased in the mDPC/shNRG group. Immunofluorescence revealed that relocation of NF-κB was similar to that of NRAGE during odontoblastic induction, in which NF-κB translocated from the cytoplasm to the nucleus.
Conclusion: NRAGE is a potent regulator of proliferation and odontoblastic differentiation of mDPCs, which might be via the NF-κB signalling pathway.
© 2015 John Wiley & Sons Ltd.
Figures




Similar articles
-
The effect of delta-like 1 homologue on the proliferation and odontoblastic differentiation in human dental pulp stem cells.Cell Prolif. 2017 Jun;50(3):e12335. doi: 10.1111/cpr.12335. Epub 2017 Feb 15. Cell Prolif. 2017. PMID: 28205268 Free PMC article.
-
The Effect of NRAGE on cell cycle and apoptosis of human dental pulp cells and MDPC-23.Int J Clin Exp Med. 2015 Jul 15;8(7):10657-67. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26379857 Free PMC article.
-
Knockdown of NRAGE induces odontogenic differentiation by activating NF-κB signaling in mouse odontoblast-like cells.Connect Tissue Res. 2019 Mar;60(2):71-84. doi: 10.1080/03008207.2018.1439484. Epub 2018 Feb 22. Connect Tissue Res. 2019. PMID: 29448842
-
Effects of homeobox gene distal-less 3 on proliferation and odontoblastic differentiation of human dental pulp cells.J Endod. 2012 Nov;38(11):1504-10. doi: 10.1016/j.joen.2012.07.009. Epub 2012 Sep 6. J Endod. 2012. PMID: 23063225
-
Effects of high-mobility group box 1 on the proliferation and odontoblastic differentiation of human dental pulp cells.Int Endod J. 2013 Dec;46(12):1153-63. doi: 10.1111/iej.12112. Epub 2013 Apr 19. Int Endod J. 2013. PMID: 23600680
Cited by
-
p75 neurotrophin receptor regulates differential mineralization of rat ectomesenchymal stem cells.Cell Prolif. 2017 Feb;50(1):e12290. doi: 10.1111/cpr.12290. Epub 2016 Sep 27. Cell Prolif. 2017. PMID: 27672006 Free PMC article.
-
The effect of delta-like 1 homologue on the proliferation and odontoblastic differentiation in human dental pulp stem cells.Cell Prolif. 2017 Jun;50(3):e12335. doi: 10.1111/cpr.12335. Epub 2017 Feb 15. Cell Prolif. 2017. PMID: 28205268 Free PMC article.
-
The Effect of NRAGE on cell cycle and apoptosis of human dental pulp cells and MDPC-23.Int J Clin Exp Med. 2015 Jul 15;8(7):10657-67. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26379857 Free PMC article.
References
-
- Sinanan AC, Hunt NP, Lewis MP (2004) Human adult craniofacial muscle‐derived cells: neural‐cell adhesion‐molecule (NCAM; CD56)‐expressing cells appear to contain multipotential stem cells. Biotechnol. Appl. Biochem. 40, 25–34. - PubMed
-
- d'Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R et al (2009) Human dental pulp stem cells: from biology to clinical applications. J. Exp. Zool. B Mol. Dev. Evol. 312B, 408–415. - PubMed
-
- Magloire H, Joffre A, Bleicher F (1996) An in vitro model of human dental pulp repair. J. Dent. Res. 75, 1971–1978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

