Glycan engagement dictates hydrocephalus induction by serotype 1 reovirus
- PMID: 25736887
- PMCID: PMC4358001
- DOI: 10.1128/mBio.02356-14
Glycan engagement dictates hydrocephalus induction by serotype 1 reovirus
Abstract
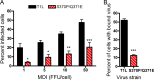
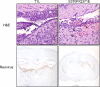
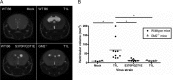
Receptors expressed on the host cell surface adhere viruses to target cells and serve as determinants of viral tropism. Several viruses bind cell surface glycans to facilitate entry, but the contribution of specific glycan moieties to viral disease is incompletely understood. Reovirus provides a tractable experimental model for studies of viral neuropathogenesis. In newborn mice, serotype 1 (T1) reovirus causes hydrocephalus, whereas serotype 3 (T3) reovirus causes encephalitis. T1 and T3 reoviruses engage distinct glycans, suggesting that glycan-binding capacity contributes to these differences in pathogenesis. Using structure-guided mutagenesis, we engineered a mutant T1 reovirus incapable of binding the T1 reovirus-specific glycan receptor, GM2. The mutant virus induced substantially less hydrocephalus than wild-type virus, an effect phenocopied by wild-type virus infection of GM2-deficient mice. In comparison to wild-type virus, yields of mutant virus were diminished in cultured ependymal cells, the cell type that lines the brain ventricles. These findings suggest that GM2 engagement targets reovirus to ependymal cells in mice and illuminate the function of glycan engagement in reovirus serotype-dependent disease.
Importance: Receptor utilization strongly influences viral disease, often dictating host range and target cell selection. Different reovirus serotypes bind to different glycans, but a precise function for these molecules in pathogenesis is unknown. We used type 1 (T1) reovirus deficient in binding the GM2 glycan and mice lacking GM2 to pinpoint a role for glycan engagement in hydrocephalus caused by T1 reovirus. This work indicates that engagement of a specific glycan can lead to infection of specific cells in the host and consequent disease at that site. Since reovirus is being developed as a vaccine vector and oncolytic agent, understanding reovirus-glycan interactions may allow manipulation of reovirus glycan-binding properties for therapeutic applications.
Copyright © 2015 Stencel-Baerenwald et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

