STAT3 Serine 727 Phosphorylation: A Relevant Target to Radiosensitize Human Glioblastoma
- PMID: 25736961
- PMCID: PMC8029431
- DOI: 10.1111/bpa.12254
STAT3 Serine 727 Phosphorylation: A Relevant Target to Radiosensitize Human Glioblastoma
Abstract
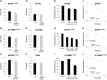
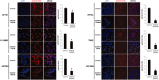
Radiotherapy is an essential component of glioma standard treatment. Glioblastomas (GBM), however, display an important radioresistance leading to tumor recurrence. To improve patient prognosis, there is a need to radiosensitize GBM cells and to circumvent the mechanisms of resistance caused by interactions between tumor cells and their microenvironment. STAT3 has been identified as a therapeutic target in glioma because of its involvement in mechanisms sustaining tumor escape to both standard treatment and immune control. Here, we studied the role of STAT3 activation on tyrosine 705 (Y705) and serine 727 (S727) in glioma radioresistance. This study explored STAT3 phosphorylation on Y705 (pSTAT3-Y705) and S727 (pSTAT3-S727) in glioma cell lines and in clinical samples. Radiosensitizing effect of STAT3 activation down-modulation by Gö6976 was explored. In a panel of 15 human glioma cell lines, we found that the level of pSTAT3-S727 was correlated to intrinsic radioresistance. Moreover, treating GBM cells with Gö6976 resulted in a highly significant radiosensitization associated to a concomitant pSTAT3-S727 down-modulation only in GBM cell lines that exhibited no or weak pSTAT3-Y705. We report the constitutive activation of STAT3-S727 in all GBM clinical samples. Targeting pSTAT3-S727 mainly in pSTAT3-Y705-negative GBM could be a relevant approach to improve radiation therapy.
Keywords: Gö6976; STAT3; glioma; radiotherapy; resistance.
© 2015 International Society of Neuropathology.
Conflict of interest statement
None.
Figures





References
-
- Amberger‐Murphy V (2009) Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets 9:381–390. - PubMed
-
- Aziz MH, Hafeez BB, Sand JM, Pierce DB, Aziz SW, Dreckschmidt NE, Verma AK (2010) Protein kinase Cε mediates Stat3Ser727 phosphorylation, Stat3‐regulated gene expression and cell invasion in various human cancer cell lines via integration with MAPK cascade (RAF‐1, MEK1/2, and ERK1/2). Oncogene 29:3100–3109. - PMC - PubMed
-
- Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19:2474–2488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

