An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM)
- PMID: 25737052
- PMCID: PMC4461864
- DOI: 10.1016/j.vetpar.2015.01.026
An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM)
Abstract
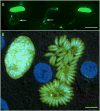
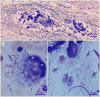
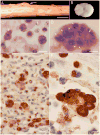
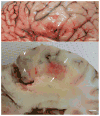
Equine protozoal myeloencephalitis (EPM) is a serious disease of horses, and its management continues to be a challenge for veterinarians. The protozoan Sarcocystis neurona is most commonly associated with EPM. S. neurona has emerged as a common cause of mortality in marine mammals, especially sea otters (Enhydra lutris). EPM-like illness has also been recorded in several other mammals, including domestic dogs and cats. This paper updates S. neurona and EPM information from the last 15 years on the advances regarding life cycle, molecular biology, epidemiology, clinical signs, diagnosis, treatment and control.
Keywords: Epidemiology; Equine protozoal myeloencephalitis; Life cycle; Marine mammals; Prevention; Sarcocystis neurona.
Published by Elsevier B.V.
Figures







References
-
- Arias M, Yeargan M, Francisco I, Dangoudoubiyam S, Becerra P, Francisco R, Sánchez-Andrade R, Paz-Silva A, Howe DK. Exposure to Sarcocystis spp. in horses from Spain determined by Western blot analysis using Sarcocystis neurona merozoites as heterologous antigen. Vet Parasitol. 2012;185:301–304. - PubMed
-
- Asmundsson IM, Dubey JP, Rosenthal BM. A genetically diverse but distinct North American population of Sarcocystis neurona includes an overrepresented clone described by 12 microsatellite alleles. Infect Genet Evol. 2006;6:352–360. - PubMed
-
- Asmundsson IM, Rosenthal BM. Isolation and characterization of microsatellite markers from Sarcocystis neurona, a causative agent of equine protozoal myeloencephalitis. Molecular Ecology Notes. 2006;6:8–10.
-
- Barr SC, Warner K. Characterization of a serine protease activity in Sarcocystis neurona merozoites. J Parasitol. 2003;89:385–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

