Applications of PET imaging with the proliferation marker [18F]-FLT
- PMID: 25737423
- PMCID: PMC4415691
Applications of PET imaging with the proliferation marker [18F]-FLT
Abstract
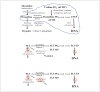
[18F]-3'-fluoro-3'-deoxythymidine (FLT) is a nucleoside-analog imaging agent for quantifying cellular proliferation that was first reported in 1998. It accumulates during the S-phase of the cell cycle through the action of cytosolic thymidine kinase, TK1. Since TK1 is primarily expressed in dividing cells, FLT uptake is essentially limited to dividing cells. Thus FLT is an effective measure of cell proliferation. FLT uptake has been shown to correlate with the more classic proliferation marker, the monoclonal antibody to Ki-67. Increased cellular proliferation is known to correlate with worse outcome in many cancers. However, the Ki-67 binding assay is performed on a sampled preparation, ex vivo, whereas FLT can be quantitatively measured in vivo using positron emission tomography (PET). FLT is an effective and quantitative marker of cell proliferation, and therefore a useful prognostic predictor in the setting of neoplastic disease. This review summarizes clinical studies from 2011 forward that used FLT-PET to assess tumor response to therapy. The paper focuses on our recommendations for a standardized clinical trial protocol and components of a report so multi center studies can be effectively conducted, and different studies can be compared. For example, since FLT is glucuronidated by the liver, and the metabolite is not transported into the cell, the plasma fraction of FLT can be significantly changed by treatment with particular drugs that deplete this enzyme, including some chemotherapy agents and pain medications. Therefore, the plasma level of metabolites should be measured to assure FLT uptake kinetics can be accurately calculated. This is important because the flux constant (KFLT) is a more accurate measure of proliferation and, by inference, a better discriminator of tumor recurrence than standardized uptake value (SUVFLT). This will allow FLT imaging to be a specific and clinically relevant prognostic predictor in the treatment of neoplastic disease.
Conflict of interest statement
Figures


References
-
- Shields AF, Lim K, Grierson J, Link J, Krohn KA. Utilization of labeled thymidine in DNA synthesis: studies for PET. J Nucl Med. 1990;31:337–42. - PubMed
-
- Shields AF, Grierson JR, Kozawa SM, Zheng M. Development of labeled thymidine analogs for imaging tumor proliferation. Nucl Med Biol. 1996;23:17–22. - PubMed
-
- Hong YS, Kim HO, Kim KP, Lee JL, Kim HJ, Lee SJ, et al. 3′-Deoxy-3′-18F-fluorothymidine PET for the early prediction of response to leucovorin, 5-fluorouracil, and oxaliplatin therapy in patients with metastatic colorectal cancer. J Nucl Med. 2013;54:1209–16. - PubMed
-
- Eriksson S, Kierdaszuk B, Munch-Petersen B, Oberg B, Johansson NG. Comparison of the substrate specificities of human thymidine kinase 1 and 2 and deoxycytidine kinase toward antiviral and cytostatic nucleoside analogs. Biochem Biophys Res Commun. 1991;176:586–92. - PubMed
-
- Munch-Petersen B, Cloos L, Tyrsted G, Eriksson S. Diverging substrate specificity of pure human thymidine kinases 1 and 2 against antiviral dideoxynucleosides. J Biol Chemistry. 1991;266:9032–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

