Quorum-sensing Salmonella selectively trigger protein expression within tumors
- PMID: 25737556
- PMCID: PMC4371937
- DOI: 10.1073/pnas.1414558112
Quorum-sensing Salmonella selectively trigger protein expression within tumors
Abstract
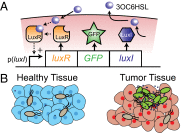
Salmonella that secrete anticancer proteins have the potential to eliminate tumors, but nonspecific expression causes damage to healthy tissue. We hypothesize that Salmonella, integrated with a density-dependent switch, would only express proteins in tightly packed colonies within tumors. To test this hypothesis, we cloned the lux quorum-sensing (QS) system and a GFP reporter into nonpathogenic Salmonella. Fluorescence and bacterial density were measured in culture and in a tumor-on-a-chip device to determine the critical density necessary to initiate expression. QS Salmonella were injected into 4T1 tumor-bearing mice to quantify GFP expression in vivo using immunofluorescence. At densities below 0.6 × 10(10) cfu/g in tumors, less than 3% of QS Salmonella expressed GFP. Above densities of 4.2 × 10(10) cfu/g, QS Salmonella had similar expression levels to constitutive controls. GFP expression by QS colonies was dependent upon the distance to neighboring bacteria. No colonies expressed GFP when the average distance to neighbors was greater than 155 µm. Calculations of autoinducer concentrations showed that expression was sigmoidally dependent on density and inversely dependent on average radial distance. Based on bacterial counts from excised tissue, the liver density (0.0079 × 10(10) cfu/g) was less than the critical density (0.11 × 10(10) cfu/g) necessary to initiate expression. QS Salmonella are a promising tool for cancer treatment that will target drugs to tumors while preventing damage to healthy tissue.
Keywords: Salmonella; bacterial anticancer therapy; cancer; localized drug delivery; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jain RK. The next frontier of molecular medicine: Delivery of therapeutics. Nat Med. 1998;4(6):655–657. - PubMed
-
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998;58(7):1408–1416. - PubMed
-
- Tannock IF, Lee CM, Tunggal JK, Cowan DSM, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8(3):878–884. - PubMed
-
- Forbes NS, Munn LL, Fukumura D, Jain RK. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 2003;63(17):5188–5193. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

