Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis
- PMID: 25737955
- PMCID: PMC4338318
- DOI: 10.1016/j.molmet.2014.12.013
Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis
Abstract
Objective: Disruption of the genes encoding either seipin or 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2) causes severe congenital generalized lipodystrophy (CGL) in humans. However, the function of seipin in adipogenesis remains poorly defined. We demonstrated recently that seipin can bind the key adipogenic phosphatidic acid (PA) phosphatase lipin 1 and that seipin forms stable dodecamers. As AGPAT2 generates PA, the substrate for lipin 1, we investigated whether seipin might bind both enzymes of this lipid biosynthetic pathway, which is required for adipogenesis to occur.
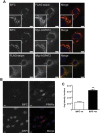
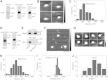
Methods: We employed co-immunoprecipitation and immunofluorescence methods to determine whether seipin can interact with AGPAT2 and the consequences of this in developing adipocytes. Atomic force microscopy was used to determine whether these interactions involved direct association of the proteins and to define the molecular architecture of these complexes.
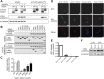
Results: Our data reveal that seipin can bind AGPAT2 during adipogenesis and that stabilizing this interaction during adipogenesis can increase the nuclear accumulation of PPARγ. Both AGPAT2 and lipin 1 can directly associate with seipin dodecamers, and a single seipin complex can simultaneously bind both AGPAT2 and lipin with a defined orientation.
Conclusions: Our study provides the first direct molecular link between seipin and AGPAT2, two proteins whose disruption causes CGL. Moreover, it provides the first example of an interaction between seipin and another protein that causally influences a key aspect of adipogenesis. Together our data suggest that the critical role of seipin in adipogenesis may involve its capacity to juxtapose important regulators of this process in a multi-protein complex.
Keywords: AGPAT2; Adipocyte; Adipogenesis; Atomic force microscopy (AFM); BSCL2; Lipid synthesis; Lipin; Lipodystrophy; Seipin.
Figures



References
-
- Agarwal A.K., Arioglu E., De Almeida S., Akkoc N., Taylor S.I., Bowcock A.M. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nature Genetics. 2002;31:21–23. - PubMed
-
- Magre J., Delepine M., Khallouf E., Gedde-Dahl T., Jr., Van Maldergem L., Sobel E. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nature Genetics. 2001;28:365–370. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

