Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens
- PMID: 25738167
- PMCID: PMC4335822
- DOI: 10.1212/NXI.0000000000000070
Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens
Abstract
Objective: To evaluate immune responses to neoantigen and recall antigens in healthy subjects treated with teriflunomide.
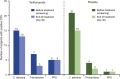
Methods: This was a randomized, double-blind, placebo-controlled study. Subjects received oral teriflunomide (70 mg once daily for 5 days followed by 14 mg once daily for 25 days) or placebo for 30 days. Antibody responses were evaluated following rabies vaccination (neoantigen) applied at days 5, 12, and 31 of the treatment period. Occurrence of delayed-type hypersensitivity (DTH) to Candida albicans, Trichophyton, and tuberculin (recall antigens) was assessed before and at the end of treatment to investigate cellular memory response. Safety and pharmacokinetics were evaluated.
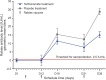
Results: Forty-six randomized subjects were treated (teriflunomide, n = 23; placebo, n = 23) and completed the rabies vaccination. Geometric mean titers for rabies antibodies were lower with teriflunomide at days 31 and 38 than with placebo. However, all subjects achieved sufficient seroprotection following rabies vaccination (titers well above the 0.5 IU/mL threshold). Overall, the DTH response to recall antigens in the teriflunomide group did not notably differ from responses in the placebo group.
Conclusions: Following vaccination, geometric mean titers for rabies antibodies were lower with teriflunomide than with placebo. However, teriflunomide did not limit the ability to achieve seroprotective titers against this neoantigen. Evaluation of DTH showed that teriflunomide had no adverse impact on the cellular memory response to recall antigens.
Classification of evidence: This study provides Class II evidence that in normal subjects treated with teriflunomide, antibody titer responses to rabies vaccination are lower than with placebo but sufficient for seroprotection.
Figures


References
-
- AUBAGIO Prescribing Information. Cambridge, MA: Genzyme Corporation, a Sanofi company; 2012.
-
- AUBAGIO Summary of Product Characteristics. Paris, France: sanofi-aventis groupe; 2013.
-
- Cherwinski HM, Cohn RG, Cheung P, et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther 1995;275:1043–1049. - PubMed
-
- Ruckemann K, Fairbanks LD, Carrey EA, et al. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem 1998;273:21682–21691. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
