Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate
- PMID: 25738172
- PMCID: PMC4335821
- DOI: 10.1212/NXI.0000000000000076
Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate
Abstract
Objective: To evaluate the influence of dimethyl fumarate (DMF, Tecfidera) treatment of multiple sclerosis (MS) on leukocyte and lymphocyte subsets.
Methods: Peripheral blood leukocyte and lymphocyte subsets, including CD3(+), CD4(+), and CD8(+) T cells; CD19(+) B cells; and CD56(+) natural killer (NK) cells, were obtained at baseline and monitored at 3 months, 6 months, and 12 months after initiation of DMF treatment.
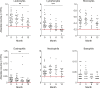
Results: Total leukocyte and lymphocyte counts diminished after 6 months of DMF therapy. At 12 months, lymphocyte counts had decreased by 50.1% (p < 0.0001) and were below the lower limit of normal (LLN) in one-half of patients. CD3(+) T lymphocyte counts fell by 44.2% (p < 0.0001). Among subsets, CD8(+) T cell counts declined by 54.6% (p < 0.0001), whereas CD4(+) T cell counts decreased by 39.2% (p = 0.0006). This disproportionate reduction of CD8(+) T cells relative to CD4(+) T cells was significant (p = 0.007) and was reflected by a 35.5% increase in the CD4/CD8 ratio (p = 0.007). A majority of CD8(+) T cell counts, but not CD4(+) T cell counts, were below the LLN even when total lymphocyte counts were greater than 500 cells/μL. CD19(+) B cell counts were reduced by 37.5% (p = 0.035). Eosinophil levels decreased by 54.1% (p = 0.006), whereas levels of neutrophils, monocytes, basophils, and NK cells were not significantly altered.
Conclusion: Subsets of peripheral blood leukocytes and lymphocytes are differentially affected by DMF treatment of MS. Reduction of CD8(+) T cells is more pronounced than that of CD4(+) T cells. These findings may have implications for cell-mediated antiviral immunity during DMF treatment.
Figures

References
-
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. - PubMed
-
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. - PubMed
-
- van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 2013;368:1658–1659. - PubMed
-
- Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013;368:1657–1658. - PubMed
-
- Sweetser MT, Dawson KT, Bozic C. Manufacturer's response to case reports of PML. N Engl J Med 2013;368:1659–1660. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
