Palladium-catalyzed enantioselective Heck alkenylation of acyclic alkenols using a redox-relay strategy
- PMID: 25738548
- PMCID: PMC4785804
- DOI: 10.1021/ja5130836
Palladium-catalyzed enantioselective Heck alkenylation of acyclic alkenols using a redox-relay strategy
Abstract
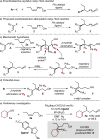
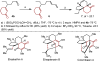
We report a highly enantioselective intermolecular Heck reaction of alkenyl triflates and acyclic primary or racemic secondary alkenols. The mild reaction conditions permit installation of a wide range of alkenyl groups at positions β, γ, or δ to a carbonyl group in high enantioselectivity. The success of this reaction is attributed to the use of electron-withdrawing alkenyl triflates, which offer selective β-hydride elimination followed by migration of the catalyst through the alkyl chain to give the alkenylated carbonyl products. The synthetic utility of the process is demonstrated by a two-step modification of a reaction product to yield a tricyclic core structure, present in various natural products.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. VCH; Weinheim: 1996.
- Nicolaou KC, Snyder SA. Classics in Total Synthesis II. More Targets, Strategies, Methods. Wiley-VCH; Weinheim: 2003.
- Saklani A, Kutty SK. Drug Discovery Today. 2008;13:161. - PubMed
- Liu KK-C, Sakya LS. Mini-Rev Med Chem. 2004;4:1105. - PubMed
-
-
For reports on asymmetric conjugate additions, see:
- Nakao Y, Chen IH, Hiyama T, Ichikawa I, Duan W, Shintani R, Hayashi T. J Am Chem Soc. 2007;129:9137. - PubMed
- Oi S, Sato T, Inoue Y. Tetrahedron Lett. 2004;45:5051.
- Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew Chem Int Ed. 2005;44:3874. - PubMed
- Hayashi T, Yamasaki K. Chem Rev. 2003;103:2829. - PubMed
-
-
-
For reports on asymmetric allylic displacement, see:
- Hamilton J, Sarlah D, Carreira EM. J Am Chem Soc. 2013;135:994. - PubMed
- Akiyama K, Gao F, Hoveyda AH. Angew Chem Int Ed. 2010;49:419. - PMC - PubMed
- Gao F, McGrath KP, Lee Y, Hoveyda AH. J Am Chem Soc. 2010;132:14315. - PMC - PubMed
- Gao F, Carr JL, Hoveyda AH. Angew Chem, Int Ed. 2012;51:6613. - PMC - PubMed
- Shintani R, Takatsu K, Takeda M, Hayashi T. Angew Chem, Int Ed. 2011;50:8656. - PubMed
- Jung B, Hoveyda AH. J Am Chem Soc. 2012;134:1490. - PMC - PubMed
-
-
-
Other methods for the enantioselective introduction of alkenyl groups, see
- Biradar DB, Gau H-M. Org Lett. 2009;11:499. - PubMed
- Pu L, Yu H-B. Chem Rev. 2001;101:757. and references within. - PubMed
- Page JP, RajanBabu TV. J Am Chem Soc. 2012;134:6556. and references within. - PMC - PubMed
- Stevens JM, MacMillan DWC. J Am Chem Soc. 2013;135:11756. and references within. - PMC - PubMed
- Cherney AH, Reisman SE. J Am Chem Soc. 2014;136:14365. - PMC - PubMed
-
-
- Dounay AB, Overman LE. Chem Rev. 2003;103:2945. and references therein. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

