Valganciclovir for symptomatic congenital cytomegalovirus disease
- PMID: 25738669
- PMCID: PMC4401811
- DOI: 10.1056/NEJMoa1404599
Valganciclovir for symptomatic congenital cytomegalovirus disease
Abstract
Background: The treatment of symptomatic congenital cytomegalovirus (CMV) disease with intravenous ganciclovir for 6 weeks has been shown to improve audiologic outcomes at 6 months, but the benefits wane over time.
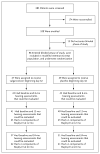
Methods: We conducted a randomized, placebo-controlled trial of valganciclovir therapy in neonates with symptomatic congenital CMV disease, comparing 6 months of therapy with 6 weeks of therapy. The primary end point was the change in hearing in the better ear ("best-ear" hearing) from baseline to 6 months. Secondary end points included the change in hearing from baseline to follow-up at 12 and 24 months and neurodevelopmental outcomes, with each end point adjusted for central nervous system involvement at baseline.
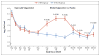
Results: A total of 96 neonates underwent randomization, of whom 86 had follow-up data at 6 months that could be evaluated. Best-ear hearing at 6 months was similar in the 6-month group and the 6-week group (2 and 3 participants, respectively, had improvement; 36 and 37 had no change; and 5 and 3 had worsening; P=0.41). Total-ear hearing (hearing in one or both ears that could be evaluated) was more likely to be improved or to remain normal at 12 months in the 6-month group than in the 6-week group (73% vs. 57%, P=0.01). The benefit in total-ear hearing was maintained at 24 months (77% vs. 64%, P=0.04). At 24 months, the 6-month group, as compared with the 6-week group, had better neurodevelopmental scores on the Bayley Scales of Infant and Toddler Development, third edition, on the language-composite component (P=0.004) and on the receptive-communication scale (P=0.003). Grade 3 or 4 neutropenia occurred in 19% of the participants during the first 6 weeks. During the next 4.5 months of the study, grade 3 or 4 neutropenia occurred in 21% of the participants in the 6-month group and in 27% of those in the 6-week group (P=0.64).
Conclusions: Treating symptomatic congenital CMV disease with valganciclovir for 6 months, as compared with 6 weeks, did not improve hearing in the short term but appeared to improve hearing and developmental outcomes modestly in the longer term. (Funded by the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov number, NCT00466817.).
Figures
Comment in
-
Valganciclovir for Congenital Cytomegalovirus.N Engl J Med. 2015 Jun 18;372(25):2463. doi: 10.1056/NEJMc1504937. N Engl J Med. 2015. PMID: 26083215 No abstract available.
-
Valganciclovir for Congenital Cytomegalovirus.N Engl J Med. 2015 Jun 18;372(25):2462. doi: 10.1056/NEJMc1504937. N Engl J Med. 2015. PMID: 26083216 No abstract available.
-
Valganciclovir for Congenital Cytomegalovirus.N Engl J Med. 2015 Jun 18;372(25):2462. doi: 10.1056/NEJMc1504937. N Engl J Med. 2015. PMID: 26083217 No abstract available.
-
Valganciclovir for Congenital Cytomegalovirus.N Engl J Med. 2015 Jun 18;372(25):2462-3. doi: 10.1056/NEJMc1504937. N Engl J Med. 2015. PMID: 26083218 No abstract available.
References
-
- Morton CC, Nance WE. Newborn hearing screening — a silent revolution. N Engl J Med. 2006;354:2151–64. - PubMed
-
- Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30. - PubMed
-
- Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–4. - PubMed
-
- Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35:226–31. - PubMed
-
- Elek SD, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet. 1974;1:1–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
